 Flocked chitosan scaffold.
Flocked chitosan scaffold.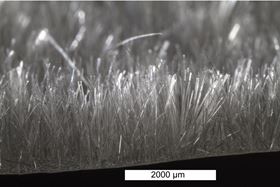 Cross section of flocked chitosan scaffold.
Cross section of flocked chitosan scaffold. Fluorescence micrograph of human mesenchymal stroma cells after 21 days of culture on/in a flock scaffold: cells are green green, while fibers show blue autofluorescence. (Courtesy of Michael Gelinsky.)
Fluorescence micrograph of human mesenchymal stroma cells after 21 days of culture on/in a flock scaffold: cells are green green, while fibers show blue autofluorescence. (Courtesy of Michael Gelinsky.)Transforming the natural material that makes up the outer shells of crustaceans like crabs, shrimps, and lobsters into a velvet-like structure could make an ideal scaffold for tissue engineering, according to researchers.
The team from the Technische Universität Dresden in Germany used only chitosan – a biopolymer derived from the exoskeletons of crustaceans – to create three-dimensional structures consisting of thin fibers stuck to a substrate [Gossla et al., Acta Biomaterialia (2016), doi: 10.1016/j.actbio.2016.08.022].
The velvet-like material is made using a well-known textile process called ‘flocking’. During the process, short fibers are charged in an electric field. When a substrate covered in glue is oppositely charged, the fibers stick on perpendicular to the surface like a carpet.
“In our study, we used self-made chitosan fibers and a viscous chitosan solution which acts as both an adhesive and flock substrate at the same time, forming a stable membrane after drying,” explains Michael Gelinsky, who led the work.
The ideal scaffold material for tissue engineering needs to be highly porous – to hold cells and allow them to grow and proliferate – and mechanically strong to support the regrowing tissue. These two properties are often mutually exclusive – but the new material strikes a good balance.
“By utilizing the textile technology of electrostatic flocking, we have developed 3D scaffolds with high and easily adjustable porosity but sufficient compressive strength,” says Gelinsky.
The idea of using flocking to create scaffolds has been explored previously, but this new material represents the first use of biocompatible and biodegradable chitosan for all the components – the substrate, the glue, and the fibers.
“We have demonstrated for the first time that applicable, fully biocompatible and biodegradable 3D scaffolds can be fabricated by flocking,” Gelinsky told Materials Today.
The researchers tested their material with two different types of cell (human mesenchymal stem cells and osteoblasts), neither of which showed any sign of toxicity. As well as its adjustable porosity and good mechanical strength, the flocked chitosan material is also highly elastic – which could be a great advantage for applications like bone or cartilage repair, where the scaffold would be subject to mechanical stresses and strains.
“In our opinion, flock scaffolds would fit best utilization in regenerative therapies for articular cartilage defects – which are still a severe clinical problem,” says Gelinsky.
The flocked chitosan scaffolds mimic cartilage very well and can be seeded with large numbers of cells suspended in a hydrogel.
Because flocking is a well-established and straightforward industrial process, it should be easy to fabricate the scaffolds at large scales, believe the researchers, added to chitosan is well accepted as a biomedical material.