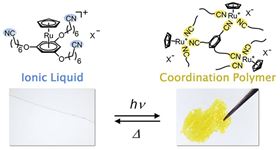
 Exposing the ionic liquid to ultraviolet light alters the chemical bonds formed by the ruthenium ions, transforming it into a yellow solid. This solid reverts to the original liquid when exposed to heat. Image: Kobe University.
Exposing the ionic liquid to ultraviolet light alters the chemical bonds formed by the ruthenium ions, transforming it into a yellow solid. This solid reverts to the original liquid when exposed to heat. Image: Kobe University.A research group from Kobe University in Japan, led by Tomoyuki Mochida, has developed a metal-containing liquid that transforms into a solid polymer when exposed to light and returns to liquid form when heated. This novel material could potentially be used for fabricating printed circuits via photolithography, among other applications, and is described in a paper in Chemical Communications.
Coordination polymers are solids with various useful applications. In recent years, research into coordination polymers has increased and scientists have developed many ways to synthesize them, but most of these methods rely on chemical reactions in solutions. Mochida and his colleagues have now come up with a way to produce these polymers by simply exposing liquids to light.
The researchers proposed that if they could control the binding process between metal ions and organic molecules using heat and light, they could create a material that drastically changes its properties when exposed to these external stimuli. This led to their creation of an ionic liquid made from a ruthenium complex with cyano groups.
Ionic liquids are salts with a melting point below 100°C, which is very low compared to standard salts such as sodium chloride. The ruthenium-based ionic liquid is colorless, clear, non-volatile and does not freeze even at -50°C. Applying ultraviolet light to the liquid for a few hours causes it to change into an amorphous coordination polymer, by altering the chemical bonds formed by the ruthenium ions, while heating this solid for one minute at 130°C causes it to return to its original ionic liquid form.
In this way, by applying light and heat, the group realized a reversible transformation between an ionic liquid and a solid coordination polymer – two substances with completely different structures and chemical properties. Being able to control the properties of materials through external stimuli such as light and heat is extremely useful for fabricating electronic components. For example, materials that solidify when exposed to light (photosensitive resins) are used for creating printed circuits, but it is currently difficult to reuse these materials.
By creating a substance that can switch between liquid and solid, the Japanese team have now produced a photosensitive resin that can be reused, and could find use in fabricating printed circuit boards, 3D printing and as an adhesive. "We plan to continue research on the molecular design of this substance, to reduce its response time, and look into creating more functions for this coordination polymer," said Mochida.
This story is adapted from material from Kobe University, with editorial changes made by Materials Today. The views expressed in this article do not necessarily represent those of Elsevier. Link to original source.