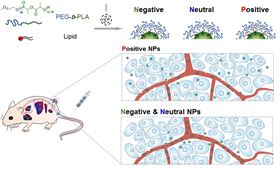
 Schematic of the formation of differently charged nanoparticles and their behavior inside a mouse model system.
Schematic of the formation of differently charged nanoparticles and their behavior inside a mouse model system.Nanoparticles could deliver anticancer drugs to tumors in the body more effectively than current medicines. To get inside tumors and be taken up by cells more readily, researchers have now found that changing the surface charge can help [H.-X. Wang et al., Nano Today (2016), doi:10.1016/j.nantod.2016.04.008].
The shape, size, and chemistry of drug-carrying nanoparticles are already known to be important factors in determining performance in physiological environments, but the effect of surface charge has been poorly understood. Now researchers from the University of Science and Technology of China in Hefei and Columbia University have made a systematic study of how surface charge affects the ability of nanoparticles to penetrate, accumulate in, and treat tumors.
‘‘We designed a lipid-polymer nanomedicine platform in which the surface charge of nanoparticles can be precisely tuned while their size and other physiochemical properties are maintained,’’ explains Kam W. Leong, who led the work along with Hong-Xia Wang, Jun Wang, and Yu-Cai Wang.
The particles, which are just 100 nm in size, were constructed from a mixture of polyethylene glycol and polylactide (PEG-b-PLA) and loaded with an anticancer drug — either docetaxel or epirubicin. Different lipid components were then added to create positive, negative, or neutral surface charge.
When tested in mouse models, the researchers found that even though positively charged (or cationic) nanoparticles show slightly inferior blood circulation time and tumor accumulation, they are much more effective at suppressing tumor growth than their negatively charged or neutral counterparts.
‘‘The efficacy of cancer nanomedicine is determined by blood circulation, tumor accumulation, tumor tissue penetration, and ultimately tumor cell internalization,’’ says Leong. ‘‘Our results reveal that the cationic PEGylated nanoparticles loaded with anticancer drugs show superior tumor treatment efficacy in five different tumor models.’’
Positively charged cationic nanoparticles appear to perform better than negatively charged or neutral counterparts because they can penetrate into tumors more easily and have a 2.5-fold higher cellular uptake. Getting into tumor cells more readily improves the nanoparticles’ ability to deliver anticancer drugs to the tumor.
‘‘This original contribution... demonstrates that cationic nanoparticles provide greater in vivo therapeutic efficacy due to increased tumor uptake and penetration,’’ comments Jackie Yi-Ru Ying of the Institute of Bioengineering and Nanotechnology in Singapore.
But the pros of positively charged nanodelivery systems — better tumor penetration and cellular uptake — must be balanced against potentially detrimental effects such as cytotoxicity and impaired colloidal stability, point out the researchers. Nevertheless, the approach could form the basis for engineering next generation nano-delivery systems for in vivo applications.
This article was originally published in Nano Today (2016), doi:10.1016/j.nantod.2016.05.001