Biodegradable implants can offer a substantial improvement in the quality of life of patients that otherwise would need a second surgery to remove the implant. To achieve this, we need to fully understand the corrosion mechanisms and degradation of the implant materials that could be used for various clinical applications. Magnesium is an ideal material in terms of invivo degradation, especially due to its natural occurrence in the human body. The excess magnesium produced during the implant degradation is excreted via the kidneys [1]. Another benefit of magnesium is the formation of a wide range of corrosion products on the surface of the implants. Most of them are calcium phosphates, very similar to the inorganic constituent of human bone, the hydroxyapatite [2]. Therefore, magnesium is an excellent candidate for bone support applications such as screws and plates.
The need for metallic biodegradable implants is not limited to large bone support applications only [3]. Recent development of magnesium wires has enabled other possible uses, such as stents, sutures, wire-reinforced composites, pulmonary artery banding or sternal fixation [4]. The main advantage of metallic wire implants is their ability to maintain the shape during the degradation. Although this may cause problems for some applications, such as wound closure sutures, in many cases it is necessary to maintain the shape and mechanical strength, as for the fixation of sternal halves after median sternotomy. Therefore, the magnesium wires could be used together with the novel biodegradable shape memory polymers [5].
There are many issues to be addressed before a magnesium wire with 250?µm in diameter can be safely employed as a sternal fixation. The necessary bending plasticity can be achieved by the use of twinning as the deformation mechanism due to the favorable microstructure and texture [4]. The mechanical strength, as well as the corrosion resistance, can be improved by alloying, for example with zinc [6]. The onset of degradation could be controlled by a biodegradable polymer coating of desired properties. Although the mechanical strength needed for safe sternal fixation for adults is currently too high for magnesium alloys, the most promising employment of magnesium-based sternal wires is for pediatric patients, where the physiological loads on sternum are much lower [7]. The use of Mg-based wires would also limit complications, such as post-sternotomy pain syndrome and further reduce surgical interventions to a minimum. This would substantially improve the quality of life of children who need invasive heart surgery.
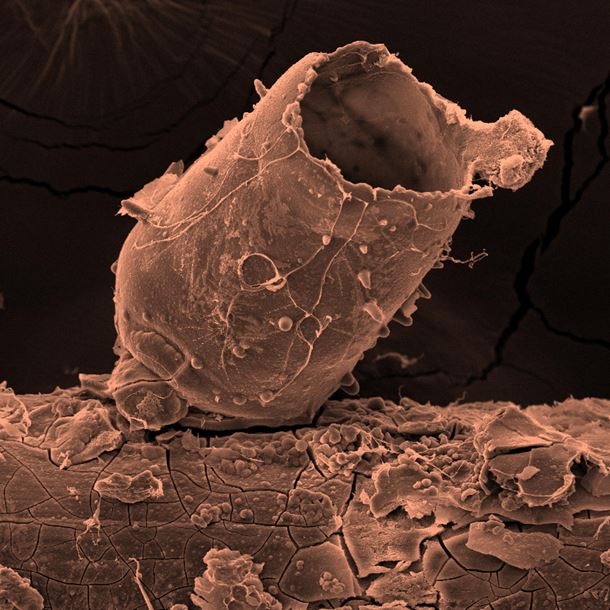
One of the key obstacles of magnesium usage as an implant is the understanding and control of the hydrogen gas generation. Hydrogen is produced during the degradation and forms bubbles on the surface of the implant. Since commercially pure magnesium is often used, the presence of hydrogen bubbles is closely connected with the iron impurities. These impurities further localize hydrogen generation via the electrochemical re-deposition of a thin iron film [8]. Once the bubble is created, the interface between the body solution and the hydrogen gas serves as the nucleation site for calcium phosphates. Depending on the rate of the hydrogen generation, the bubble is either fully enclosed by calcium phosphates or the constrained bubble grows and forms an open tube, as shown in the image. At the initial stage, the calcium phosphate layer is transparent, flexible and rich in calcium. Focused ion beam cross-sectioning revealed that after this initial state, the layer thickens and the phosphorus content increases rapidly. This is connected with the brittle fracture behavior of these hollow shapes. For thin magnesium wires, this localization of hydrogen can be a problem for several reasons. The loss of mechanical integrity as the outcome of local corrosion is the main problem. A possible solution could be sought by using ultra-pure magnesium. However, even ultra-pure magnesium tends to corrode locally, according to our results. The other option is to design the polymer coating in a way that the localization of hydrogen generation could be minimized. The use of thinner wires made into ropes can also lower the risk of the critical loss of mechanical integrity due to the localized corrosion.
The cover image of this issue depicts a SEM image of a growing calcium orthophosphate tube on top of the commercially pure magnesium wire with a diameter of 250?µm. This particular wire degraded for 48 hours in the alpha modification of Minimal Essential Medium (αMEM) with fetal bovine serum and antibiotics. A thin gold layer was deposited on this particular sample to enable better conductivity. A scanning electron microscope Quanta 450 was used for this image. The width of the micrograph is 300?µm. It is necessary to mention that the cracks in the calcium phosphate layer on the wire are at least partially caused by the vacuum exposure during the sample preparation and observation. An additional observation by 3D optical microscopy or laser scanning confocal microscopy is always necessary to correctly interpret the information provided by electron microscopy on such samples. The closed or partially closed morphological features provide an obstacle for tissue healing and are therefore very undesirable as described above.
However, it is worth to mention, that the hydrogen generation invivo is limited by the complex dynamics of a living body environment. An in vivo study or a non-static model of simulated body environment is needed to fully understand the kinetics of nucleation on the interface between the hydrogen gas and the body solution. The recent results reported in this issue will contribute to the ongoing development of biodegradable magnesium-based wires for invasive heart surgery and hopefully will result in fewer complications connected with the need for multiple surgeries and other postoperative complications such as persistent anterior chest wall pain.
Acknowledgements
Financial support of the Czech Technical University in Prague in the frame of the project SGS18/191/OHK4/3T/14 and financial support of the European Regional Development Fund (project CZ.02.1.01/0.0/0.0/16-019/0000778) is gratefully acknowledged. The authors would like to sincerely thank L. Borecká for SEM operation and A. Jancová, M. Krížková, J. Manák, Z. Sucharda and M. Žaloudková for technical assistance.
Further reading
[1]W. Jahnen-Dechent, M. Ketteler
Clin. Kidney J., 5 (Suppl_1) (2012), pp. i3-i14, 10.1093/ndtplus/sfr163
CrossRefView Record in Scopus
[2]M. Zuo, et al.
Mater. Lett., 240 (2019), pp. 279-283, 10.1016/j.matlet.2019.01.001
ArticleDownload PDFView Record in Scopus
[3]H.-S. Han, et al.
Mater. Today, 23 (2019), pp. 57-71, 10.1016/j.mattod.2018.05.018
ArticleDownload PDFView Record in Scopus
[4]A. Jäger, S. Habr, K. Tesar
Mater. Design, 110 (2016), pp. 895-902, 10.1016/j.matdes.2016.08.016
ArticleDownload PDFView Record in Scopus
[5]G.I. Peterson, A.V. Dobrynin, M.L. Becker
Adv. Healthcare Mater., 6 (21) (2017), pp. 1-16, 10.1002/adhm.201700694
1700694
CrossRef
[6]M. Nemec, et al.
Mater. Character., 134 (2017), pp. 69-75, 10.1016/j.matchar.2017.10.017
ArticleDownload PDFView Record in Scopus
[7]W.E. McGregor, D.R. Trumble, J.A. Magovern
J. Thorac. Cardiovasc. Surg., 117 (6) (1999), pp. 1144-1150, 10.1016/S0022-5223(99)70251-5
ArticleDownload PDFView Record in Scopus
[8]D. Höche, et al.
Phys. Chem. Chem. Phys., 18 (2016), pp. 1279-1291, 10.1039/C5CP05577F
CrossRefView Record in Scopus