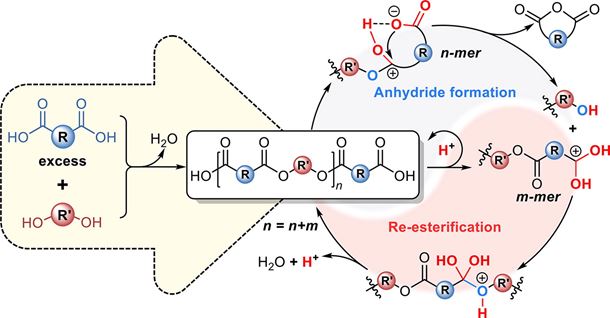
Abstract: Most commodity polyesters are synthesized via melt polycondensation of dicarboxylic acid and diol using metallic catalysts; however, the resultant metal residues can pose toxic effects on human and environment. Although polyesters can be synthesized through autocatalysis of dicarboxylic acid without additional catalysts, high molecular weight (HMW) products cannot be obtained by this strategy, which was previously attributed to the low equilibrium constant of esterification and the difficulty of removing water. Herein, we get a new understanding that the kinetic deviation of dicarboxylic acid/diol monomers is the only reason for the low molecular weight of polyesters by autocatalysis. Accordingly, we introduce a dynamic stoichiometric strategy to overcome this difficulty using anhydride-formable dicarboxylic acids as monomers through a tandem mechanism involving proton transfer, anhydride formation and re-esterification. A series of catalyst-free HMW polyesters, including poly(butylene succinate) (PBS), poly(ethylene succinate) (PES), poly(butylene succinate-co-butylene adipate) (PBSA), and poly(ethylene succinate-co-ethylene terephthalate) (PEST), were thereby synthesized. This new approach not only enables large-scale production of HMW polyesters comparable to commercial products, but also avoids the problems associated with catalysts, which is very promising for the applications with high safety requirements.
Read full text on ScienceDirect
DOI: 10.1016/j.mattod.2021.07.024
Share this journal article