Metal-free carbon-based catalysts design for oxygen reduction reaction towards hydrogen peroxide: From 3D to 0D
Volume 63, Issue , Page 339–359
| Ellen Mitchell, Xunyu Lu, Dewei Chu, Lu Shang, Tierui Zhang, Rose Amal, Zhaojun Han
Abstract
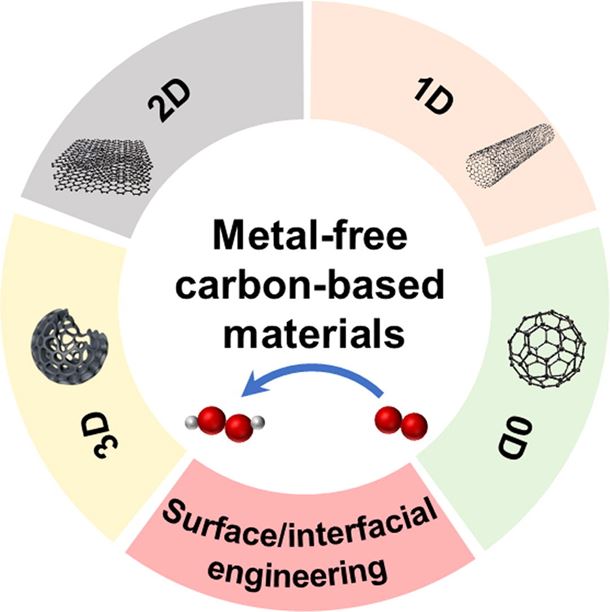
Hydrogen peroxide (H2O2) is a versatile and environmentally-friendly oxidant with widespread industrial applications. In recent years, electrochemical production of H2O2 through the two-electron oxygen reduction reaction (ORR) pathway has been considered a promising alternative to the energy-intensive anthraquinone process. Among various electrocatalysts proposed for electrochemical generation of H2O2, metal-free carbon-based materials have attracted significant attention due to their low cost and large abundance. In this review, recent progress made in different types of metal-free carbon-based catalysts is presented. The fundamental aspects of the ORR mechanism and methodologies of ORR performance evaluation are introduced. Different metal-free electrocatalysts are then reviewed based on their dimensions, including three-dimensional, two-dimensional, one-dimensional, and zero-dimensional (3D, 2D, 1D, and 0D, respectively) catalysts. Various strategies, such as heteroatom doping, structural engineering, and defect engineering are examined for their role in enhancing catalytic efficiency. Furthermore, surface and interfacial engineering for achieving high H2O2 production is discussed. Lastly, challenges and opportunities in this field are proposed to guide future research toward the rational design of metal-free catalysts for the electrochemical production of H2O2.
See full text for more information.
Read full text on ScienceDirect
DOI: 10.1016/j.mattod.2023.02.004
Share this journal article