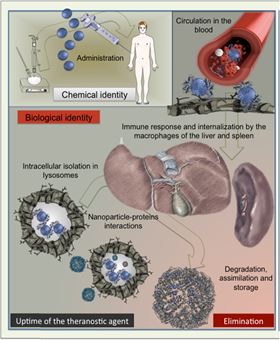
 Figure 1. Schematic view of nanoparticles lifecycle after intravenous administration.
Figure 1. Schematic view of nanoparticles lifecycle after intravenous administration.![Figure 2. Multiscale follow-up of iron oxide nanocubes over time using in vivo MRI in mice, ex vivo EPR quantification in organs, TEM observations of intracellular distribution and morphological biotransformations [17].](//assets.materialstoday.com/wpimg/float/c1707d42-9ba2-44dd-8a41-06f3b478dcdf.jpeg) Figure 2. Multiscale follow-up of iron oxide nanocubes over time using in vivo MRI in mice, ex vivo EPR quantification in organs, TEM observations of intracellular distribution and morphological biotransformations [17].
Figure 2. Multiscale follow-up of iron oxide nanocubes over time using in vivo MRI in mice, ex vivo EPR quantification in organs, TEM observations of intracellular distribution and morphological biotransformations [17].Exposure to nanoparticles (NPs) and the development of nanomedicines justifies an exhaustive assessment of the fate and potential toxicity of nanomaterials in vivo. While acute effects of NPs, evaluated by exposure/response assays, are sometimes described, the transformations of nanomaterials inflicted by the biological environment are much less investigated.
Decreasing in size to the nanoscale endows materials with new properties while enhancing particles' reactivity. NP processing in vivo includes biotransformation, degradation, bio-assimilation, elimination or simply persistence – processes orchestrated by complex and dynamic interactions with various components of biological media, crossed by NPs during their odyssey throughout the organism ( Fig. 1). Recent studies show that biological interactions continuously remodel the identity and properties of NPs [1]. The molecules within biological fluids severely reshape the surface of NPs [2] and [3] and initiate particle aggregation, opsonization or enzymatic attack and degradation [4]. Such remodeling may regulate the transport of NPs in physiological media, cellular internalization and potential toxicity [5]. The main difficulty is to characterize and follow complex interactions between NPs and the biological milieu in situ.
Another crucial factor influencing the fate of NPs is the time spent within the organism. NPs are transported and transformed through kinetic processes that are slow. One of the main fears concerning NPs is related to their potential long-time persistence within the body, which could elicit chronic inflammatory reactions to foreign materials. Such apprehensions are likely inherited from asbestos-related diseases, emerging decades after exposure.
Henceforth, the central issue becomes the lifecycle of NPs in the body from initial exposure to complete elimination or assimilation. Indeed, at times we should prefer reactive, rapidly degradable NPs, while at others long-term, inert and persistent NPs will be required to reside “inactive” in the organism. On one hand, the degradation processes of NPs and their by-products may cause unexpected biological reactions, while on the other hand, accumulation of non-degradable NPs might saturate lysosomal compartments and perturb degradative and autophagic pathways that are essential for cells to degrade proteins [6].
Surprisingly, while chemists can easily handle NP synthesis and tune size, shape, organization and properties, exploration of the lifecycle of NPs in vivo is still in its infancy. Undeniably, many studies investigate the behavior of NPs in the first hours or days after exposure, but rarely focus on periods of months or years after administration, which can be necessary for the body to eliminate or degrade particles. The lack of time for comprehensive investigations (publish or perish) partly explains this fact, but methodological difficulties to trace rare NPs in vivo, over long periods of time, are also involved. Accordingly, specific methodologies should be developed to detect and quantify nanomaterials, their speciation and residues in biological tissues, and characterize their morphological modifications at relevant scales over time.
Here we describe the evaluation of the lifecycle of inorganic magnetic iron oxide nanoparticles (IONPs), a promising multifunctional class of inorganic nanoparticles for diagnostic and therapeutic applications, and summarize the methodologies that have been designed to follow their time-dependent biodistribution, transformation and degradation in vivo ( Fig. 2).
If the biological environment remodels the particles, how do their magnetic properties evolve? How long will the particles be detectable by magnetic resonance imaging (MRI) or be able to heat the tumor? What are the recycling processes of such exogenous nanomaterials? Obviously, a compromise should be reached with particles design, balancing long-term degradability and innocuousness with sufficiently long-lasting therapeutic efficiency.
The biodistribution of NPs, including macrophage capture, is affected by protein remodeling [3]. Magnetic separation allows specific extraction of magnetic NPs from complex biological media such as blood, fluids and sub-cellular compartments and allows the evaluation of the corona, which is acquired across particles journey in vivo. Importantly, the corona varies over time depending on the stage of cell processing [7]. The IONP potential of orienting in the direction of the magnetic field can be used to probe the proteins associated with IONPs specifically, independently of free biomolecules [8]. Indeed, protein adsorption essentially depends on surface coating.
However, the composition of the corona differs when IONPs are incubated in a medium with 10% of plasma (generally used for cell culture) or in pure plasma, which resembles in vivo conditions. This important result questions the relevance of in vitro tests for toxicity assessments and emphasizes the difficulty of mimicking NP behavior in vivo. Moreover, some proteins (e.g. albumin and apolipoprotein) have a stabilizing effect, while others, such as fibrinogen, trigger particle aggregation. Remarkably, the populations of differently encompassed particles coexist in plasma and are differently processed by immune cells. Consequently, macrophages do not capture these populations in the same manner or at the same time.
The tendency of NPs to aggregate in biological media (due to the loss of coating, the adsorption of host's biomolecules or because of active biological processes, such as cellular internalization in endocytic compartments) also impacts the magnetic behavior of IONPs [9]. In subcellular compartments, IONPs have less freedom to rotate and translate, and experience dipole–dipole magnetic interactions. Their magnetic susceptibility diminishes and the temperature of transition between superparamagnetic and ferromagnetic behavior increases. Consequently, the heating ability of IONPs under an alternating magnetic field might decrease when internalized by tumor cells [10]. The extracellular distribution of particles and their ability to heat outside the cells, but within the tumoral stroma [11], are particularly favorable for tumor hyperthermia. Contrariwise, the sub-compartment confinement of IONPs is advantageous for cell detection by MRI [12], non-invasive in vivo tracking of cell migration [13] and cell manipulation by magnetic forces [14].
As the prerequisite for NPs use is their long-term innocuousness, the elementary questions remain if/how nanoparticles degrade, what are their degradation products and if degradation products can be assimilated, recycled or eliminated. To monitor the long-term fate of IONPs over time, MRI provides a powerful and unrivaled method (Fig. 2). Yet the quantification remains challenging since MR relaxivity significantly depends on the local environment and physical state of particles, which varies with their transformations in the organism.
Elemental analysis, the gold standard method for quantifying inorganic particles, is inappropriate if used alone for IONPs quantification, due to the high amount of endogenous iron. To distinguish superparamagnetic IONPs from endogenous non-magnetic iron forms, we could rely on nanomagnetism methods such as electron paramagnetic resonance (EPR), which provides a specific and sensitive quantification technique for IONPs [15] (Fig. 2).
IONPs of different sizes, shapes, organization (spheres, cubes or heterostructures with IONPs associated to gold particles) and coating (glucose-derivative, polyethylene glycol (PEG) and amphiphilic polymer) have been tracked over one year after intravenous administration (2.5 mg/kg bw) with multiscale methods associating MRI follow-up, EPR quantification and electron microscopy [11],[15], [16], [17]. As expected, these particles mainly reach the liver and the spleen, depending on the nature of IONPs coating. Moreover EPR reveals the coating-dependent elimination of superparamagnetic iron from these organs occurring months after injection. Concomitantly, with the disappearance of superparamagnetic iron, an increase in the non-magnetic iron pool is measured by elemental analysis, suggesting local dissolution of nanoparticles in liver and spleen and their transformation into non-magnetic iron [15].
In parallel with such macroscopic follow-up, it is important to observe the evolution of subcellular distribution and morphology of IONPs at the atomic scale using transmission electron microscopy (TEM) techniques (Fig. 2). IONPs are mainly found as clusters within lysosomes of splenic and hepatic macrophages one day post-injection and tend to sort in the periphery or electron dense areas of the lysosomes at later times [17].
The transformation of gold–iron oxide heterostructures definitively proves the local degradation of iron oxide nanocrystals within the lysosomes: when iron oxide dissolves, the more persistent gold moieties remain intact and play the role of tracers for iron oxide transformation [16]. Moreover IONPs become increasingly surrounded by ferritin proteins, which are rich in iron, but present different atomic structures, as differentiated by high resolution TEM [15], [17] (Fig. 2). Ferritins regulate and store the iron in a safe form, in order to provide it to the body when needed and avoid the deleterious effect of labile iron species [18]. The coexistence of ferritins situated proximally to IONPs suggests a mechanism of local iron transfer from degraded IONPs to endogenous iron storing proteins. The processes of iron oxide crystal degradation, loss of superparamagnetic properties, remediation by endogenous iron storage proteins and iron recycling ensure the innocuousness of IONPs.
Importantly, the degradability of NPs in vivo can be controlled. In order to screen the degradation patterns of IONPs, the same particles were tracked over their whole lifecycle in a medium mimicking the acidic pH of lysosomes and the effect of iron chelators[19], [20] (Fig. 2). In agreement with liver and spleen samples, where crystalline residues are frequently observed, the degradation process of IONPs is a step-by-step corrosion governed by surface reaction mechanisms. The morphological alterations of IONPs and the evolution of their magnetic properties show how the surface coating and the particle's architecture affect degradation [17], [19].
For example, multicore flower-shaped IONPs monocrystals, formed by coalescence of magnetically-oriented iron oxide seeds, exhibit a cooperative magnetic behavior and exceptional properties for hyperthermia and MRI [21]. Unlike other “monobloc” nanoparticles, nanoflowers rapidly disintegrate into smaller and therapeutically much less effective constituents. Remarkably, the disintegration of nanoflowers can be more or less delayed by a layer of gold, depending on the thickness and porosity of the gold shell. Polymer layers can also be employed for similar protective strategies.
Generally, the efficiency of organic or inorganic nanoshields relies on their ability to prevent the access of cellular media to the iron-oxide surface. The importance of polymeric coverings was illustrated by the preferential degradation of nanocube vertexes where the surface coating is less dense when compared to the edges [17]. In line with nanoscale analysis, magnetic properties of nanocubes or gold–iron oxide nanohybrids are more persistent if the particles are coated with a double-chained amphiphilic polymer than with hydrophilic chains of PEG, a difference which is also observed in vivo in spleen and liver one year post-injection [11], [16], [17]. Hence the in vivo degradability of nanostructures may be modulated by the design of composite materials, core–shell heterostructures and appropriate coating.
Another key factor in the degradability of IONPs is the aggregation state. Generally, isolated IONPs erode faster than aggregated ones. This suggests that the cell's tendency to individualize particles within the lysosomes in vivo is an efficient way to trigger/accelerate degradation [17]. Despite the non-ambiguous observation of local degradation of NPs, resilient intact particles are still observed even one year after injection, regardless of their nature, demonstrating the persistence of a minority of particles [11].
What triggers the dissolution of one particle compared to another is still an open question. This problem could greatly benefit from recent advances of TEM in the liquid environment, which allows observation of the interactions between nanoparticles and wet cells [22].
We have described key stages of lifecycle monitoring of NPs in organisms and presented principal methods to assess biotransformations of magnetic NPs at relevant scales. Associating the study of NP lifecycle with biodistribution and potential toxicity will allow a better understanding and anticipation of the relationship between NP fate and safety, while optimizing therapeutic efficiency.
Acknowledgements
*Corresponding authors at: Université Paris Diderot, Laboratoire Matière et Systèmes Complexes and Laboratoire Matériaux et Phénomènes Quantiques, 10 rue Alice Domon et Léonie Duquet, 75205 Paris Cedex 13, France.
This paper was originally published in Nano Today 11 (3) (2016), doi: 10.1016/j.nantod.2015.10.001
References
1. G.V. Lowry, K.B. Gregory, S.C. Apte, J.R. Lead. Environ. Sci. Technol., 46 (2012), pp. 6893–6899
2. B. Fadeel, N. Feliu, C. Vogt, A.M. Abdelmonem, W.J. Parak. Wiley Interdisciplin. Rev.: Nanomed. Nanobiotechnol., 5 (2013), pp. 111–129
3. M.P. Monopoli, C. Aberg, A. Salvati, K.A. Dawson. Nat. Nano., 7 (2012), pp. 779–786
4. S.J. Soenen, W.J. Parak, J. Rejman, B. Manshian. Chem. Rev., 115 (2015), pp. 2109–2135
5. S. Loeve, B.B. Vincent, F. Gazeau. Nano Today, 8 (2013), pp. 560–565
6. S. Stern, P. Adiseshaiah, R. Crist. Part. Fibre Toxicol., 9 (2012), p. 20
7. F. Bertoli, G.-L. Davies, M.P. Monopoli, M. Moloney, Y.K. Gun’ko, A. Salvati, et al. Small, 10 (2014), pp. 3307–3315
8. L. Lartigue. ACS Nano, 6 (2012), pp. 2665–2678
9. M. Levy, C. Wilhelm, N. Luciani, V. Devaux, F. Gendron, A. Luciani, et al. Nanoscale, 3 (2011), pp. 4402–4410
10. R. Di Corato, A. Espinosa, L. Lartigue, M. Tharaud, S. Chat, T. Pellegrino, et al. Biomaterials, 35 (2014), pp. 6400–6411
11. J. Kolosnjaj-Tabi, R. Di Corato, L. Lartigue, I. Marangon, P. Guardia, A.K.A. Silva, et al. ACS Nano, 8 (2014), pp. 4268–4283
12. P. Smirnov, F. Gazeau, J.C. Beloeil, B.T. Doan, C. Wilhelm, B. Gillet. Contrast Media Mol. Imaging, 1 (2006), pp. 165–174
13. P. Smirnov, M. Poirier-Quinot, C. Wilhelm, E. Lavergne, J.C. Ginefri, B. Combadiere, et al. Magn. Reson. Med., 60 (2008), pp. 1292–1297
14. D. Fayol, G. Frasca, C. Le Visage, F. Gazeau, N. Luciani, C. Wilhelm. Adv. Mater., 25 (2013), pp. 2611–2616
15. M. Levy, N. Luciani, D. Alloyeau, D. Elgrabli, V. Deveaux, C. Pechoux, et al. Biomaterials, 32 (2011), pp. 3988–3999
16. J. Kolosnjaj-Tabi, Y. Javed, L. Lartigue, J. Volatron, D. Elgrabli, I. Marangon, et al. ACS Nano, 9 (2015), pp. 7925–7939
17. L. Lartigue, D. Alloyeau, J. Kolosnjaj-Tabi, Y. Javed, P. Guardia, A. Riedinger, et al. ACS Nano, 7 (2013), pp. 3939–3952
18. C. Beaumont, C. Delaby. Semin. Hematol., 46 (2009), pp. 328–338
19. M. Levy, F. Lagarde, V.A. Maraloiu, M.G. Blanchin, F. Gendron, C. Wilhelm, et al. Nanotechnology, 21 (2010), p. 395103
20. A.S. Arbab, L.B. Wilson, P. Ashari, E.K. Jordan, B.K. Lewis, J.A. Frank. NMR Biomed., 18 (2005), pp. 383–389
21. Y. Javed, L. Lartigue, P. Hugounenq, Q.L. Vuong, Y. Gossuin, R. Bazzi, et al. Small, 10 (2014), pp. 3325–3337
22. N.d. Jonge, D.B. Peckys, G.J. Kremers, D.W. Piston. Proc. Natl. Acad. Sci. U.S.A., 106 (2009), pp. 2159–2164