Minerals are an integral component of living organisms. Over sixty different biominerals(1) have been identified in animals, endowing tissues with a range of functions, in particular, support (e.g. skeleton), defence (e.g. turtle shell), and feeding (e.g. teeth). Biominerals, however, are not restricted to hard tissues; they are also commonly found in soft tissues. For example, otoconia (calcium carbonate crystals) are an essential part of the otolithic membrane, which is located in the vestibular system of the inner ear of vertebrates(2) and plays a critical role in the brain’s interpretation of balance.
The skin of several animals, including fish, amphibians, non-avian reptiles, avian reptiles, and mammals(3) also contains minerals in the form of scales or osteoderms, while a mineralised structure named the sclerotic ring is found in the eyes of several groups of vertebrates.(4) Mineralisation of soft tissues in mammals has predominantly been attributed to pathological processes. Mineral formation has been reported in the context of many diseases, including the majority of cardiovascular diseases, all cancer types, (5,6) Alzheimer’s disease, (7) schizophrenia, (8) age-related macular degenerative disease, (9) and infectious diseases, such as tuberculosis(10) and HIV(11) (examples can be seen in Fig. 1).
 Figure 1: a Density-dependent colour scanning electron micrograph (DDC-SEM) of breast tissue, showing calcium deposits, where purple represents inorganic material. Scale bar = 1 µm. b DDC-SEM of an aortic valve, showing spherical particles of calcium phosphate, where orange represents inorganic material and green organic material. Scale bar = 10 µm. c DDC-SEM of placenta tissue, showing calcium deposits, where red represents inorganic material and turquoise organic material. Scale bar = 10 µm. d DDC-SEM of a kidney stone, where purple/pink represents inorganic material12. Scale bar = 2 µm
Figure 1: a Density-dependent colour scanning electron micrograph (DDC-SEM) of breast tissue, showing calcium deposits, where purple represents inorganic material. Scale bar = 1 µm. b DDC-SEM of an aortic valve, showing spherical particles of calcium phosphate, where orange represents inorganic material and green organic material. Scale bar = 10 µm. c DDC-SEM of placenta tissue, showing calcium deposits, where red represents inorganic material and turquoise organic material. Scale bar = 10 µm. d DDC-SEM of a kidney stone, where purple/pink represents inorganic material12. Scale bar = 2 µmTraditionally, the biominerals present in hard tissues has mainly been characterised by biophysical techniques. The nature, origins, mechanism of formation and function of minerals have been investigated by electron microscopy,(9,13) diffraction techniques (X-ray and electron diffractometry), (14) and spectroscopic methods. (5,15) However, in-depth physicochemical characterisation studies of biominerals in soft tissues remain elusive thus far.
Moreover, research on the pathophysiological processes leading to the formation of minerals in soft tissues has mainly focussed on biochemical mechanisms, motivated by the idea that biochemistry can explain physicochemical mechanisms of mineralisation. These minerals have been thought of as a disease by-product disease and biologically inert, therefore their significance has long been underestimated and underappreciated.
However, minerals are known to affect cellular signalling; for example, implantation of biomaterials made of calcium phosphate minerals in soft tissues drives bone formation through recruitment and/or transdifferentiation of local or circulating cells, which subsequently adopt a mineralising phenotype.(16) Moreover, it has further been established that substrate stiffness plays a crucial role in cellular behaviour(17) Therefore the presence of minerals in soft tissues, such as the brain (one of the softest tissues in the body), certainly alters its mechanical properties, which in turn affect cellular signalling and behaviour. Pathological minerals can also dissolve in the organic extracellular matrix, altering the properties and surface of the matrix, which has a substantial impact on cell behaviour. (18) Besides, in the case of calcium phosphate minerals, the increase in calcium and phosphate concentration around a dissolving mineral affects surrounding cells. (18) Finally, calcium phosphate nanoparticles have been proven to be able to enter cells and are commonly used to carry DNA fragments and transfect cells. (19)
Given the vital role, minerals can have in cell and tissue biology; it is fundamental to be able to understand their origins and formation mechanisms. Moreover, the presence of a specific mineral can provide valuable information about (patho)biological mechanisms, and reveal the processes that control and guide mineral formation towards a specific mineral phase. From a physicochemical perspective, individual minerals are created under strict conditions, at which the concentration of ions, temperature, pH, and pressure cause the formation of one mineral phase. However, in a biological system, proteins can alter mineralisation processes to favour the production of one mineral over another. (20) These complex mineralisation processes involve the interplay between cellular, extracellular and mineral components. By determining the composition of a mineral in a pathological mineralisation site, information can be gained on the characteristics of the biological microenvironment, including cells and proteins. For example, urinary track stones(21) elemental composition can be correlated to some of the most abundant ions present in cells or microenvironment in the organism.
Mineral crystallographic characteristics can also indicate the specific biochemical pathway responsible for their formation. A highly crystalline mineral is formed in a highly-controlled system, in which proteins take a central role in the mineralisation mechanism (as is commonly the case for shells and other minerals present in molluscs(22)). For example, in the case of molluscs shell and pearls, protein sheets control the nucleation of calcite crystals in specific positions and orientations, which then undergo a sudden transition to form the final structure. (22)
The morphology, size and shape of a mineral can give important clues about the microenvironment. A constricting environment would force the mineral into shapes, such as the case of bone mineral where the inorganic material is deposited in the spaces between collagen fibres. (23) Additionally, a deviation from a mineral’s natural habit suggests internal modifications or constriction by the biological matrix. Synthetic crystalline hydroxyapatite, for instance, is usually observed as a rhombohedral habit.(24) In ocular and other mineralising diseases, it has however been reported as a spherical particle;(9,13) hinting on a vesicle being a possible nucleation site.
The architecture of a mineral at the nanoscale and the characterisation of proteins and cell organelles interacting with it can also reveal insights on the progression of a disease, as e.g. in age-related macular degeneration.(9) For example, an analysis of the minerals observed in the retina unveiled the presence of previously unknown, distinct calcified structures, and has allowed to identify that hydroxyapatite is associated to more advanced disease stages while has also allowed for the identification of a mineral called whitlockite. (9)
In analogy to other ‘omics’ strategies, a mineralomics approach will focus on the collection of all information that is available on a biological system, including physicochemical information about minerals in the tissue, is considered fundamental to understand tissue processes. Most of the currently available ‘omics’ approaches focus exclusively on organic molecules and structures. It is expected that in analogy to other ‘omics’ approaches are now commonly applied for a variety of molecules, including lipids, proteins or metabolites, which enabled important discoveries of structure-function relationships, mineralomics can provide similarly important new insights. More importantly, mineralomics can will be integrated into multi-omics approaches, enabling a comprehensive analysis of samples and all their constituents.
In contrast to ‘omics’ approaches for organic molecules, mineralomics does not require the development of new methods but envisages a different workflow and research strategy (Fig. 2), in which the study of minerals in tissues and cells is integrated into biological and medical research. In addition to biochemical methods, minerals are identified and characterised to establish connections between mineral and the biological components of the system.
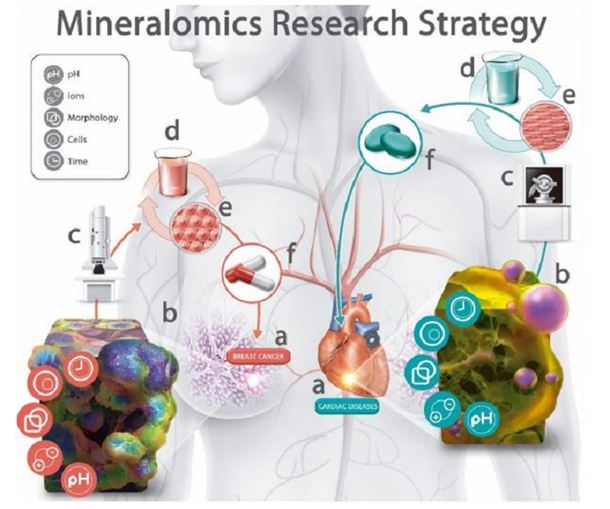
Figure 2: The Mineralomics research strategy for the analysis of tissues and the development of treatments and prevention methods for diseases associated with mineral formation in soft tissues. a Disease presenting mineral formation in affected soft tissue, for example, breast cancer and cardiac diseases. b Mineral formation is a consequence of the interaction of different chemical and biological players, such as pH, ionic concentration and composition, microenvironment responsible for the morphology of the mineral formed, cells present and/or responsible for mineral formation, and time length during which the mineral is formed. c Physico-chemical characterisation of minerals present in tissues, providing important indications of the microenvironment, cells and mechanism responsible for mineral formation. d, e In vitro chemical and biological studies to confirm the origins, mechanism of formation and prevention methods for the formation of minerals in soft tissues. f Translation of the information gained from biophysicochemical research into the development, diagnosis, prevention and treatment methods of diseases in a.
An excellent example of the mineralomics research approach can be seen in the study of cardiovascular calcification. The cardiovascular system is one of the most common sites of pathological mineralisation, and therefore, mineralomics can be applied to reveal the underlying mechanisms of cardiovascular diseases. The paradigm for cardiovascular mineralisation has long been that the mineral stems from pathological bone formation in cardiovascular tissue. (25) However, using a mineralomics approach and applying advanced electron microscopy and elemental analysis,(13) we could show that cardiovascular mineralisation can be distinct from bone formation. The mineralised regions in cardiovascular tissue are mainly composed of micro minerals in the form of spherical particles (Fig. 3) – a morphology not found in bone. Similarly, a mineralomics approach has been applied to understand extracellular-vesicle-derived microcalcification in atherosclerotic plaques, in a study where the minerals produced by an in vitro mineralisation model were compared to the minerals found in carotid arteries.(26) These studies again suggest that the mechanism of mineral formation in vascular tissue is different from mechanisms of bone formation and that other cells than bone cells may be responsible for mineralisation.
 Figure 3: Density-dependent colour scanning electron micrograph of an aortic valve, showing spherical particles of calcium phosphate. 27 Scale bar = 2 µm.
Figure 3: Density-dependent colour scanning electron micrograph of an aortic valve, showing spherical particles of calcium phosphate. 27 Scale bar = 2 µm.By the same approach, it has also been demonstrated that vascular minerals are formed from calcium phosphate and considerable amounts of magnesium;(13) by contrast, bone and teeth mineral is formed from calcium and phosphorus. The presence of different elements in vascular minerals again supports that these minerals do not follow the same mechanism of formation as bone and teeth. Moreover, the same results suggest that cells that contain a high concentration of magnesium might also play a role in vascular mineralisation. Finally, the mineral characterisation also revealed that vascular mineral particles are formed from minerals that diffract as highly crystalline single crystals, suggesting an initially slow mechanism of formation or possibly controlled by proteins, as in the case of biominerals with complex morphologies that diffract as single crystals formed by molluscs.
Taking all into account would like to stress again that mineralomics would enable the study of pathological minerals, which have long been underestimated in their power to predict and provide valuable information about diseases and its mechanisms. Physico-chemical characterisation methods, are readily available, simple to apply and often more cost-effective than biochemical methods. By applying these physicochemical characterisation methods and by considering the biological and medical context, mineralomics can provide a holistic understanding of the characteristics and role of minerals in the human body, and particularly in diseases. Mineralomics has the potential to provide critical insights into human (patho)biology and to contribute considerably to medicine, especially when integrated into a multi-omics research.
References
1. Ginebra CAMP. Biomineralisation and Biomaterials: Woodhead Publishing; 2015.
2. Beisel KW, Wang-Lundberg Y, Maklad A, Fritzsch B. Development and evolution of the vestibular sensory apparatus of the mammalian ear. Journal of vestibular research : equilibrium & orientation. 2005;15(5-6):225-41.
3. Yang W, Chen IH, Gludovatz B, Zimmermann EA, Ritchie RO, Meyers MA. Natural flexible dermal armor. Adv Mater. 2013;25(1):31-48.
4. Atkins JB, Franz-Odendaal TA. The sclerotic ring of squamates: an evo-devo-eco perspective. J Anat. 2016;229(4):503-13.
5. Baker R, Rogers KD, Shepherd N, Stone N. New relationships between breast microcalcifications and cancer. British journal of cancer. 2010;103(7):1034-9. 6. Smolski M, Turo R, Whiteside S, Bromage S, Collins GN. Prevalence of prostatic calcification subtypes and association with prostate cancer. Urology. 2015;85(1):178-81.
7. Forstl H, Burns A, Cairns N, Luthert P, Levy R. Basal ganlia mineralisation in Alzheimer’s disease - A Comparative study of clinical, neuroradiological and neurropathological finding. Behavioural Neurology. 1992;5(1):53-7.
8. Sandyk R, Kay SR. Abnormal EEG and calcification of the pineal gland in schizophrenia. Int J Neurosci. 1992;62(1-2):107-11.
9. Tan ACS, Pilgrim MG, Fearn S et al. Calcified nodules in retinal drusen are associated with disease progression in age-related macular degeneration. Science translational medicine. 2018;10(466).
10. De Vuyst D, Vanhoenacker F, Gielen J et al. Imaging features of musculoskeletal tuberculosis. Eur Radiol. 2003;13:1809-1819.
11. Rezaeian P, Miller PE, Haberlen SA et al. Extra-coronary calcification (aortic valve calcification, mitral annular calcification, aortic valve ring calcification and thoracic aortic calcification) in HIV seropositive and seronegative men: Multicenter AIDS Cohort Study. J Cardiovasc Comput Tomogr. 2016;10(3):229-36. 8
12. Bazin D, Jouanneau C, Bertazzo Set al. Combining field effect scanning electron microscopy, deep UV fluorescence, Raman, classical and synchrotron radiation Fourier transform Infra-Red Spectroscopy in the study of crystal-containing kidney biopsies. Comptes Rendus Chimie. 2016;19(11- 12):1439-50.
13. Bertazzo S, Gentleman E, Cloyd KL, Chester AH, Yacoub MH, Stevens MM. Nano-analytical electron microscopy reveals fundamental insights into human cardiovascular tissue calcification. Nat Mater. 2013;12(6):576-83.
14. Thompson RB, Reffatto V, Bundy JG et al. identification of hydroxyapatite spherules provides new insight into subretinal pigment epithelial deposit formation in the aging eye. Proceedings of the National Academy of Sciences of the United States of America. 2015;112(5):1565-70.
15. Singh VK, Rai PK. Kidney stone analysis techniques and the role of major and trace elements on their pathogenesis: a review. Biophys Rev. 2014;6(3-4):291-310.
16. Ducheyne P, Qiu Q. Bioactive ceramics: the effect of surface reactivity on bone formation and bone cell function. Biomaterials. 1999;20(23-24):2287-303. 17. Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix Elasticity Directs Stem Cell Lineage Specification. Cell. 2006;126(4):677-89.
18. Bertazzo S, Zambuzzi WF, Campos DDP, Ogeda TL, Ferreira CV, Bertran CA. Hydroxyapatite surface solubility and effect on cell adhesion. Colloids and Surfaces B: Biointerfaces. 2010;78(2):177- 84.
19. Whiteside TL, Gambotto A, Albers A, Stanson J, Cohen EP. Human tumor-derived genomic DNA transduced into a recipient cell induces tumor-specific immune responses. Proceedings of the National Academy of Sciences. 2002;99(14):9415.
20. Abou Neel EA, Aljabo A, Strange A et al. Demineralization-remineralization dynamics in teeth and bone. Int J Nanomedicine. 2016;11:4743-63.
21. Ratkalkar VN, Kleinman JG. Mechanisms of Stone Formation. Clin Rev Bone Miner Metab. 2011;9(3-4):187-97.
22. Belcher AM, Wu XH, Christensen RJ, Hansma PK, Stucky GD, Morse DE. Control of crystal phase switching and orientation by soluble mollusc-shell proteins. Nature. 1996;381(6577):56-8.
23. Blair HC, Larrouture QC, Li Y et al. Osteoblast Differentiation and Bone Matrix Formation In Vivo and In Vitro. Tissue Eng Part B Rev. 2017;23(3):268-80. 24. Jang HL, Jin K, Lee J et al. Revisiting Whitlockite, the Second Most Abundant Biomineral in Bone: Nanocrystal Synthesis in Physiologically Relevant Conditions and Biocompatibility Evaluation. ACS Nano. 2014;8(1):634-41.
25. Sage AP, Tintut Y, Demer LL. Regulatory mechanisms in vascular calcification. Nat Rev Cardiol. 2010;7(9):528-36.
26. Hutcheson JD, Goettsch C, Bertazzo S et al. Genesis and growth of extracellular-vesicle-derived microcalcification in atherosclerotic plaques. Nature Materials. 2016;15:335. 27. Bertazzo S, Steele JAM, Chester AH, Yacoub MH, Stevens MM. Cardiovascular calcification violet pearl. The Lancet. 2014;384(9950).