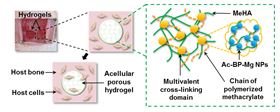
 Schematic of the HA-BP-Mg nanocomposite hydrogel structure and in-situ bone regeneration process. Left: schematic of the infiltration and migration of host cells in HA-BP-Mg nanocomposite hydrogels. Green box: schematic of the multivalent crosslinking micro-domains formed by clustered Ac-BP-Mg NPs, which stabilize the hydrogels.
Schematic of the HA-BP-Mg nanocomposite hydrogel structure and in-situ bone regeneration process. Left: schematic of the infiltration and migration of host cells in HA-BP-Mg nanocomposite hydrogels. Green box: schematic of the multivalent crosslinking micro-domains formed by clustered Ac-BP-Mg NPs, which stabilize the hydrogels.Researchers have developed a novel nanocomposite based on a hydrogel laced with metal nanoparticles that could support the repair of damaged or diseased bone [Zhang et al., Acta Biomaterialia 64 (2017) 389].
Hydrogels are attractive for many clinical applications because of their versatile physical and bioactive properties. Metal ions, meanwhile, such as Mg2+, have been found to encourage cell adhesion and differentiation, stimulating local bone formation and growth. The team from the Chinese University of Hong Kong brought together these two materials to create a novel biomaterial able to deliver Mg2+ ions in a controlled manner.
“We developed a novel bioactive nanocomposite hydrogel based on hyaluronic acid and self-assembled bisphosphonate-magnesium nanoparticles,” explains Liming Bian. “The hydrogel exhibits enhanced mechanical properties, improved capacity for mineralization, and controlled release kinetics of Mg2+.”
The team found that the hydrogels facilitate in vivo bone regeneration by releasing Mg2+ ions, which enhance cell adhesion and spreading while promoting the differentiation of human mesenchymal stem cells (hMSCs). Because the nanocomposite is simply based on an acellular hyaluronic acid hydrogel impregnated with bisphosphonate-magnesium (BP-Mg) nanoparticles, the approach greatly simplifies the regenerative therapy.
The novel nanocomposite is fabricated by mixing methacrylated hyaluronic acid (MeHA), acrylated bisphosphonate and MgCl2. BP-Mg nanoparticles are formed bearing acrylate groups, which crosslink and strengthen the hydrogel network.
“The porous structure of our hydrogels facilitates the migration of the host cells into the hydrogels,” points out Bian. “Meanwhile, the Mg2+ released from hydrogels may not only enhances the cell-matrix interaction, facilitating cell migration and adhesion, but also promotes osteogenesis of the adhered cells.”
As the hydrogels degrade gradually over time, Mg2+ ions are released in a controlled manner to facilitate the adhesion and spreading of stem cells. Moreover, the degradation of the nanocomposite is an added benefit because its presence does not hinder subsequent bone growth.
“To the best of our knowledge, no previous reports have demonstrated hydrogels able to achieve the loading and sustained release of Mg2+,” says Bian. “Mg ions have been shown to facilitate cell adhesion and differentiation and stimulate local bone formation and healing. But the sustained and controlled delivery of magnesium ions by the biomaterial implants has remained challenging.”
The team believes that their approach could represent a universal platform for the delivery of other bioactive ions such as zinc or strontium. Multiple species of different metal cations could be loaded into the hydrogel simultaneously and released as a bioactive ‘cocktail’.
The nanocomposite hydrogel is now being trialed in large animal models and the researchers are collaborating with other groups to develop a bioink that could enable the printing of precise three-dimensional structures.