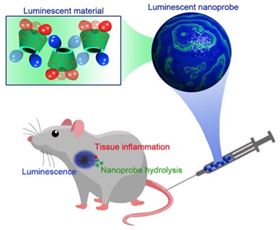
 Design of a myeloperoxidase (MPO)-responsive, biodegradable, and luminescent material and nanoparticle based on functionalized cyclodextrin.
Design of a myeloperoxidase (MPO)-responsive, biodegradable, and luminescent material and nanoparticle based on functionalized cyclodextrin.Nanoparticles made from a luminescent, biodegradable material could enable inflammatory diseases to be imaged in real-time, according to researchers [Guo et al., Materials Today (2017), doi: 10.1016/j.mattod.2017.09.003].
Inflammation is a key feature of disorders such as diabetes and is implicated in many other diseases from arthritis to cardiovascular disease to cancer. A type of white blood cells known as neutrophils play a central role in the body’s inflammatory response and initiate chronic inflammatory diseases. The ability to detect, track, and quantify neutrophils in the body could provide a much-needed boost to the diagnosis and treatment of inflammatory diseases.
Researchers from the Third Military Medical University and Zhejiang University in China, and the University of Chicago think they may have come up with a way to do just that in the form of nanoparticles derived from ring-shaped sugar molecules (cyclodextrin) functionalized with a luminol, a small luminescent molecular probe.
The functionalized cyclodextrin nanoparticles are responsive to an enzyme expressed by neutrophils called myeloperoxidase or MPO. In cell culture tests using neutrophils derived from mice showing an inflammatory response, the nanoparticles show strong and sustained luminescence.
Similarly, when administered to mice with various inflammatory conditions, the nanoparticle probe showed strong, stable and prolonged luminescence when triggered by the tell tale biochemical markers of inflammation, elevated levels of MPO and reactive oxygen species.
Not only does the approach allow neutrophils to be imaged in real-time, the intensity of the luminescent signal can also be correlated with the actual amount of neutrophils.
“The nanoprobe shows desirable luminescence for the detection of different inflammatory disorders in both superficial and deep tissues, enabling noninvasive and real-time imaging of inflammation-associated diseases,” says Jianxiang Zhang. “As activated neutrophils in different inflammatory disorders can be selectively imaged using the nanoprobe, the initiation, progression, and resolution of inflammation can be detected.”
Tests of the safety and biocompatibility of the MPO-responsive material threw up no issues, according to the researchers, either in its native or nanoparticle form. More importantly, the MPO-responsive material can be completely broken down into smaller biochemical molecules in the body and excreted.
The team now plans to evaluate how the nanoprobe works with chronic inflammatory disorders such as pulmonary disease, cancer, and atherosclerosis.
“We will also explore strategies that can enhance tissue penetration capability and inflammation targeting capacity in future studies,” Zhang told Materials Today.
The MPO-responsive nanoparticles could also be used to deliver therapeutics or contrast agents or screen for new anti-inflammatory agents, he adds.