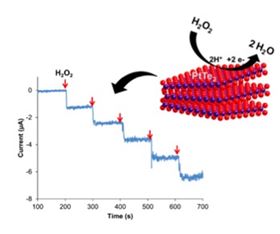
 Schematic illustration of synthesized layered Pt dichalcogenide detecting hydrogen peroxide.
Schematic illustration of synthesized layered Pt dichalcogenide detecting hydrogen peroxide.A new material for electrochemical sensing of hydrogen peroxide based on chalcogens, which include sulfur, selenium, tellurium, and a tiny amount of platinum promises outstanding performance, according to researchers [Rohaizad et al., Applied Materials Today 19 (2020) 100606, https://doi.org/10.1016/j.apmt.2020.100606].
Platinum’s prowess in catalysis and electrocatalysis, as well as its resistance to corrosion, has made it ideal for a broad range of applications from catalytic converters in vehicles and industrial catalysts to anticancer drugs and data storage. But its scarcity and high cost limit large-scale, long-term use. Much effort has been dedicated to finding ways of using less platinum while still benefitting from its unique attributes.
Now a team from Nanyang Technological University, University of Chemistry and Technology in Prague, and Brno University of Technology has combined Pt with the chalcogens S, Se, and Te to create layered compounds with electrochemical properties. The compounds are synthesized by simply heating stoichiometric amounts of the respective elements together. The layered structure of the resulting transition metal dichalcogenide (TMCs) creates a large surface area and is held together by covalent-like bonding instead of typical van der Waals’. Consequently, platinum dichalcogenides can be semiconducting (PtS2), semi-metallic (PtSe2), or metallic (PtTe2).
“The metallic nature of layered PtTe2 [is an] attribute not shared with its counterparts [and] is probably the reason for its high electrocatalytic activity for efficiently reducing H2O2,” explains Martin Pumera, who led the effort.
One of the functions of hydrogen peroxide, H2O2, is as an important messenger in cellular signaling and physiological pathways. It acts as a biomarker of reactive oxygen species (ROS), which are overproduced in living systems under ‘oxidative stress’ caused by cardiovascular and neurodegenerative diseases, including Alzheimer’s. Detecting H2O2 could, therefore, be a useful indicator of these conditions.
“In the diagnostic field, one important issue to avoid is the instability of the bioreceptors used in biosensor technology,” point out Pumera and his team. “We used PtTe2 for H2O2 sensing because we realized that this material is able to mimic the peroxidase activity and replace enzymes such as Horseradish peroxidase and Catalase.”
The team found that the inherent electrochemistry of metallic PtTe2 is superior to the other platinum dichalcogenides in detecting H2O2.
“Our PtTe2-base sensor can be used to develop a highly sensitive and inexpensive point-of-care H2O2 detection system,” says Pumera.
The fabrication of platinum dichalcogenides is efficient and reproducible, and the material is stable for a long time. While platinum is scarce and expensive, only very small amounts are needed for each sensor. The researchers are now exploring mass production of the sensor using cheap, paper-based disposable substrates.