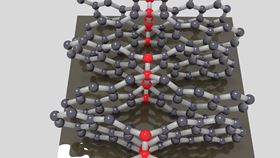
 Compressed glassy carbon contains buckled graphene sheets (blue spheres) connected at nodes with diamond-like bonding (red spheres). (Credit: Timothy Strobel.)
Compressed glassy carbon contains buckled graphene sheets (blue spheres) connected at nodes with diamond-like bonding (red spheres). (Credit: Timothy Strobel.) Credit: Timothy Strobel.
Credit: Timothy Strobel.Carbon comes in a variety of different flavors from soft graphite to ultrahard diamond. Now researchers have created a new form of carbon that combines some of the best attributes of each in a single material [Hu et al., Sci. Adv. 3 (2017) e1603213].
It is the chemical bonds in carbon, notably its unique ability to have sp, sp2 and sp3 bonding states, which gives rise to its different forms. Pressure can be used to control the type of chemical bonding and induce phase transformations from one form to another. Under extreme pressure carbon takes the form of superhard insulating diamond, which is dominated by sp3 bonding. In contrast, graphene, which is dominated by sp2 bonding, is a flexible 2D Dirac semimetal. Forms of carbon that combine both sp2 and sp3 bonding phases promise the best of both worlds in terms of mechanical and electrical properties.
The team from Yanshan University and Center for High Pressure Science and Technology Advanced Research in China, Carnegie Institution of Washington, the University of Chicago, and Pennsylvania State University took a form known as ‘glassy carbon’ and subjected it to high pressures and temperatures in a process is similar to that used to transform graphite into diamond. But in a new twist, the researchers kept the temperature just below that needed to produce diamond.
“The resulting compressed glassy carbon exhibits exceptional hybrid properties in that it is lightweight, ultrastrong, very hard, elastic, and electrically conductive,” says Zhisheng Zhao of Yanshan University.
Compressed glassy carbon contains domains of interpenetrating graphene planes that form lattices with open pores on the angstrom scale. From this, the team fabricated millimeter-scale pillars that show promising properties. The material has a hardness of up to 28 GPa, compared with just 5 GPa for ‘raw’ glassy carbon, and an exceptional compressive strength of 9 GPa. Compressed glassy carbon also shows high levels of elasticity in response to local deformation.
“In simple terms, the material combines the best properties of graphitic- and diamond-like forms of carbon,” says Zhao. “We are excited about the prospects of creating new materials by starting from disordered, metastable states. Our ultimate goal is to obtain extremely strong and superhard materials with superelasticity.”
This combination of properties could be useful for many potential applications, such as military armor and aerospace, point out the researchers.
“This paper presents an interesting new approach to strongly increase the compressive strength of glassy carbon by a factor up to 9 GPa, as well as its Young modulus and hardness with a moderate density increase,” says Nicola Pugno of the University of Trento, Italy. “Applications where ‘lightweight’ materials are required to sustain high pressures can be envisioned,” he adds.
The team is now working on using the same approach to generate other new structural materials, while also synthesizing larger samples on the centimeter-scale.
This article was originally published in Nano Today (2017), doi: 10.1016/j.nantod.2017.06.004