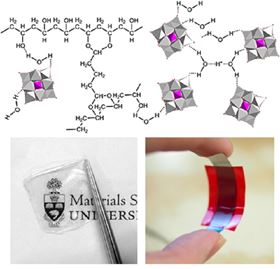
 Schematic of proton conduction bridge formation between heteropolyacids (HPA) within a cross-linked polyvinyl alcohol (PVA) host (Top); photographs of a HPA-PVA polymer electrolyte film and its enabled solid flexible supercapacitor (Bottom).
Schematic of proton conduction bridge formation between heteropolyacids (HPA) within a cross-linked polyvinyl alcohol (PVA) host (Top); photographs of a HPA-PVA polymer electrolyte film and its enabled solid flexible supercapacitor (Bottom).The Materials for Energy Session Best Contribution Award presented by Steve Zinkle goes to Han Gao, University of Toronto, Canada for the oral presentation ‘Advanced proton conducting polymer electrolytes and their applications in solid supercapacitors’
As a winner of the above Materials Today Asia Contribution Award, Han Gao and colleagues discuss their work with us.
In recent years, significant efforts have been dedicated to flexible supercapacitors, mainly targeting consumer electronics, micro-electronics, and wearable or printable electronics to supplement or replace batteries. Although thin and/or free-standing supercapacitor electrodes [1] as well as flexible substrates [2] have led to significant advances in such devices, there are still obstacles to their commercialization. One of the main shortcomings is the liquid electrolytes, which limit the form factors of the device and require extra volume and weight for sealing and packaging.
Polymer electrolytes, acting as separator and ionic conductor, are ideal candidates for the next generation flexible supercapacitors. An ideal high-performance polymer electrolyte should exhibit: (i) high ionic conductivity; (ii) good ion accessibility at the electrode\electrolyte interface; (iii) wide electrochemical stability window; and (iv) high environmental and temperature stability. Although the conductivity of polymer electrolytes is typically a few orders of magnitude lower than that of their liquid counterparts, deploying them in the form of thin films can mitigate this issue and provide high performance in a supercapacitor device.
The most mature polymer electrolyte is Nafion® and its derivatives. Their low environmental stability and high cost have restricted the application at the ambient conditions for supercapacitors. Alternatively, acid/polymer blends of liquid acids (e.g. H2SO4 or H3PO4) and polymers (e.g. polyvinyl alcohol (PVA)) are currently the most widely used polymer electrolytes for flexible supercapacitors due to their ease of preparation and good environmental stability. Nonetheless this system still exhibit low proton conductivity especially at low relative humidity (RH) environment.
Heteropoly acids (HPA) are promising low-cost solid proton conductors. Their high proton conductivity are due to two major factors: (i) highly hydrogen-bonded conduction pathways in the crystal lattice, and (ii) dynamic dissociation of co-crystallized water molecules in the crystal hydrate. The former is the result of a large number of crystallized water molecules in the crystal hydrate (e.g. silicotungstic acid, SiWA•nH2O), leading to “quasi-liquid” states [3]. The latter occurs via the interactions with oxygen atoms of the Keggin anions and thus increases the density of free protons. The protons, in the forms of H+-nH2O clusters (e.g. H3O+ or H5O2+), are transferred by hopping from H+-nH2O donor sites to nH2O acceptors within the HPA, yielding high solid-state proton conductivity (e.g. 0.027 Scm-1 for SiWA•28H2O at room temperature) [4]. However HPA powders cannot easily form films and earlier studies relied on HPAs pressed into pellets, leading to inhomogeneous particle contact [5, 6], and consequently limiting their applications as solid electrolytes for supercapacitors.
Fortunately, this issue can be addressed by developing HPA-polymer composite electrolytes that can leverage the excellent film forming capability of the polymer and the high proton conductivity of HPA. A polymer-in-salt electrolyte system using SiWA and PVA has been developed (see figure). A systematic approach has been used to improve the performance of SiWA-PVA polymer electrolytes through additives and polymer structural modifications. The electrolytes showed excellent proton conductivity, environmental stability, and film flexibility that enables thin and light weight solid supercapacitors. They outperformed Nafion® in terms of environmental stability at ambient conditions [7].
These SiWA-based polymer electrolytes have demonstrated ultra-high rate performance in both electrochemical double layer capacitors and pseudo-capacitors. The solid polymer electrolyte-enabled devices can charge and discharge at up to 100 Vs-1, with a time constant of 10 ms [8, 9]. Due to the redox of the Keggin anion (i.e. SiW12O404-) at negative potentials, the potential window of the SiWA-based polymer electrolyte system is limited. To further expand the potential window of the SiWA-based electrolyte, and hence increase the energy density, alternative HPAs with different central heteroatoms were synthesized and applied to the HPA-PVA electrolyte system. For example, tungstoboric acid, H5BW12O40•nH2O (BWA), can achieve a comparable potential window to H2SO4, much wider than that of SiWA. Furthermore, the BWA-based polymer electrolyte also demonstrated higher solid-state proton conductivity.
To better understand the enhancement in conductivity of the BWA-based polymer electrolyte especially at the low RH condition, proton conduction properties of the polymer electrolyte were deconvoluted [10] and the results suggested a higher proton density and mobility of BWA-PVA electrolyte than that of SiWA-based electrolyte. The higher proton density was mainly due to the increased number of protons associated with each Keggin anion by replacing Si with B. In addition, unlike SiWA, BWA retained more co-crystallized water molecules in their crystal lattice, providing more proton conduction ‘pathways’. These results all suggest that BWA is a very promising ionic conductor in HPA-PVA polymer electrolytes for enhanced power and energy density of the solid supercapacitors.
There are many promising chemistries for flexible solid supercapacitors, but scale-up and commercialization still need to overcome many hurdles. The current rapid pace of development in high performance materials coupled with expertise in system integration will facilitate the realization of next generation supercapacitors. The development of any polymer electrolyte requires a thorough understanding of the mechanism and interactions affecting ion transport. The insights from this work will not only contribute to the development of supercapacitors, but can also be extended to other electrochemical devices such as sensors, printed electronics, electrochemical displays, fuel cells, and batteries.
[1] T. Chen, L. Dai, J. Mater. Chem. A, 2 (2014) 10756-10775.
[2] X. Cai, M. Peng, X. Yu, Y. Fu, D. Zou, J. Mater. Chem. C, 2 (2014) 1184-1200.
[3] U.B. Mioc, M.R. Todorovic, M. Davidovic, P. Colomban, I. Holclajtner-Antunovic, Solid State Ionics, 176 (2005) 3005-3017.
[4] K.D. Kreuer, M. Hampele, K. Dolde, A. Rabenau, Solid State Ionics, 28–30, Part 1 (1988) 589-593.
[5] R.C.T. Slade, J. Barker, H.A. Pressman, Solid State Ionics, 28–30, Part 1 (1988) 594-600.
[6] R.C.T. Slade, H.A. Pressman, E. Skou, Solid State Ionics, 38 (1990) 207-211.
[7] H. Gao, H. Wu, K. Lian, Electrochem. Commun., 17 (2012) 48-51.
[8] H. Gao, K. Lian, J. Mater. Chem., 22 (2012) 21272-21278.
[9] H. Gao, Y.-J. Ting, N.P. Kherani, K. Lian, J. Power Sources, 222 (2013) 301-304.
[10] H. Gao, K. Lian, ACS Appl. Mater. Interfaces, 6 (2013) 464-472.
Han Gao, Alvin Virya, and Keryn Lian
Department of Materials Science and Engineering, University of Toronto, Canada