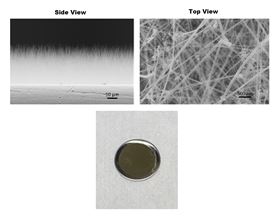
 (Clockwise from top left) Photos of silicon nanowires grown on a stainless-steel disk shown in side, top and macroscopic views. The disk is about the size of a quarter. Image: Los Alamos National Laboratory.
(Clockwise from top left) Photos of silicon nanowires grown on a stainless-steel disk shown in side, top and macroscopic views. The disk is about the size of a quarter. Image: Los Alamos National Laboratory.In silicon-wire lithium-ion batteries, the electrolyte can carve away the silicon, blocking electron pathways and greatly diminishing the charging capacity of these promising devices. Now, in a paper in Nature Nanotechnology, researchers report how their detailed investigations of this process could open up fresh research avenues for finally harnessing the great potential of silicon for revolutionizing high-capacity, long-lasting batteries for everything from cell phones to automobiles.
“With this new understanding, we propose to improve silicon nanowire lithium-ion battery performance by developing a coating approach that isolates the silicon from the electrolyte,” said Jinkyoung Yoo, a Los Alamos National Laboratory staff scientist and a corresponding author of the paper. Yoo is a semiconductor nanomaterial grower in the Center for Integrated Technologies (CINT), a US Department of Energy user facility at Los Alamos and Sandia National Laboratories.
Industry and national laboratory researchers alike consider silicon to be the most promising high-capacity anode material for practical application in next-generation lithium-ion batteries. Batteries comprise anodes, which bring electrons in, and cathodes, which move them out to generate current.
Lithium-ion batteries with graphite-based anodes have provided the power for long-lasting cell phones and electric vehicles with more than 400 miles of driving range. Developing the next generation of batteries with silicon anodes, which are known to have 10 times more storage capacity than batteries with graphite anodes, has been stymied by fading capacity after repeated charging.
After 100 charging/discharging cycles, a battery using silicon can only retain 60% of its original storage capacity—not good enough for everyday technology. And no one knew exactly why – until now.
In early applications, when silicon spherical particles were exposed to the electrolyte and charged, they expanded 300% and destroyed the anode. In all types of batteries, the process of exposing the anode to the electrolyte creates a reaction that forms the solid-electrolyte interphase (SEI), which is essential for electrochemical reactions in batteries and governs battery stability. When the SEI detaches from the anode, as it does with silicon, electrical contact breaks and the battery capacity goes down.
“We had once thought that nanowires would solve the problem of silicon expanding in the electrolyte, because a wire can stretch in length, but it turns out we didn’t understand what is happening,” Yoo explained.
To find out more, Yoo and his colleagues grew a 'forest' of silicon nanowires on a stainless-steel disk as the anode for their battery experiment. The CINT facility at Los Alamos has a unique capability for growing this kind of silicon wire directly on an anode. They then combined cryogenic scanning transmission electron microscopy with an advanced analysis algorithm to reveal in 3D the correlated structural and chemical evolution of the silicon nanowires and the SEI that forms on them.
This revealed that the electrolyte penetrates the silicon nanowires everywhere, forming pockets of SEI that disrupt electron pathways. The process ends up creating isolated islands of silicon in the anode that cannot contribute to battery capacity. The next research step, according to Yoo, is to try coating silicon particles or nanowires to preserve the integrity of the silicon in the presence of the electrolyte.
This story is adapted from material from Los Alamos National Laboratory, with editorial changes made by Materials Today. The views expressed in this article do not necessarily represent those of Elsevier. Link to original source.