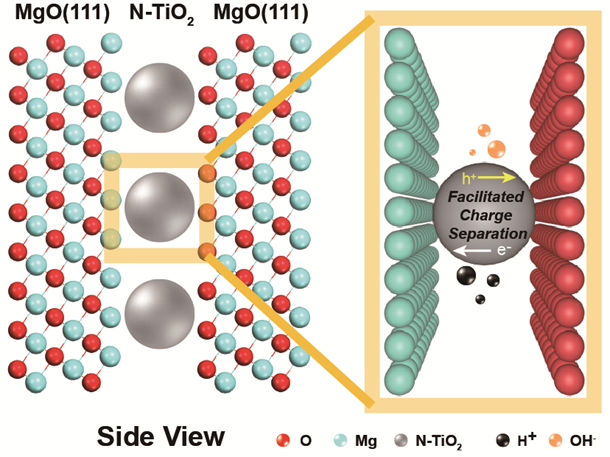
 Schematic diagram of the photocatalyst and location of the key charge separation event which splits water. Yiyang Li, University of Oxford (2020)
Schematic diagram of the photocatalyst and location of the key charge separation event which splits water. Yiyang Li, University of Oxford (2020)Raising the temperature can allow photocatalysts to use the sun’s energy to split water more effectively.
A promising option for sustainable energy would be to use sunlight to split water into hydrogen and oxygen. These products can then be recombined, releasing energy and water. Achieving this enticing fuel cycle in practice, however, requires finding efficient and robust photocatalysts to absorb light and facilitate the key water-splitting reaction.
Yiyang Li and Edman Tsang at the University of Oxford, UK, review progress towards this aim in the journal Materials Today Sustainability. “The key to enhancing the photocatalytic performance is to improve the light absorption,” says Tsang, “but in our own research we noticed that higher light absorption does not necessarily lead to better catalytic activities.”
This observation motivated the authors to focus on recent studies offering novel strategies for improving photocatalytic performance. One particularly promising insight, identified by the authors in their own work, is that catalysts operating at higher temperatures promote the key water-splitting reaction more effectively.
Solar energy is trapped when it serves to separate particles with different electric charges within the catalytic system. The higher energy ‘photo-exciton’ state generated by this fundamental process drives the water-splitting reactions. The efficiency depends on limiting the tendency for the separated charges to recombine.
Raising the temperature to the region of 270 degrees Celsius suppresses this recombination. “This novel strategy could open a new direction for innovations in this field,” says Tsang.
The authors emphasize that hydrogen gas generated by water splitting offers the cleanest chemical fuel for mankind. Water and sunlight are abundant and freely available, and the operation of the cyclic splitting and recombination process is itself pollution-free. There are challenges in the transport and storage of hydrogen fuel, but these are steadily being addressed and met by ongoing research.
The work by Tsang’s own research group began by modifying the structure of semiconductor crystals composed of magnesium, nitrogen, titanium and oxygen. This led them to demonstrate a laboratory-scale water-splitting system at elevated temperatures that is at least 100 times as efficient as most results reported by other researchers.
Although the authors review other developments in the fast-moving research endeavour, they believe that highlighting their own discoveries will offer other researchers assistance in their work.
Tsang explains that improving the overall efficiency of the systems is the key to making water splitting a practical industrial-scale energy capture and storage technology. Considerable progress is being made, but more is required to meet the desired targets, such as that set by the US Department of Energy of a hydrogen price in the 2–4 US dollar range.
“This will require efficiencies of 10 per cent,” says Tsang, in contrast with the one per cent achieved by the most efficient systems demonstrated so far. By further developing innovations, including those considered in this review, the authors believe this to be a realistic goal for the future.
Article details:
Li, Y. & Tsang, S.C.E. “Recent progress and strategies for enhancing photocatalytic water splitting,” Materials Today Sustainability (2020)