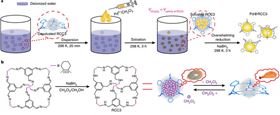
 (a) Schematic of the encapsulation process that creates Pd nanoclusters inside an RCC3 matrix. (b) Reduction of CC3R to RCC3 cage using NaBH4. Desolvated RCC3 looses porosity as the flexible cage cavities collapse but can be restored to its original crystalline state by adding a few drops of CH2Cl2.
(a) Schematic of the encapsulation process that creates Pd nanoclusters inside an RCC3 matrix. (b) Reduction of CC3R to RCC3 cage using NaBH4. Desolvated RCC3 looses porosity as the flexible cage cavities collapse but can be restored to its original crystalline state by adding a few drops of CH2Cl2.Catalysts provide a surface for chemical reactions to take place, the greater the surface the better the catalyst. Nanostructured metals make ideal catalysts and there has been much interest in nanoclusters in particular because of their remarkable catalytic properties. But, in practice, metal nanocluster catalysts can suffer from various drawbacks such as aggregation, leaching, and irregular distribution on or weak attachment to support materials.
To get around these problems, researchers from the National Institute of Advanced Industrial Science and Technology in Osaka and Kobe University in Japan, and Yangzhou University in China have come up with a clever solution. Qiang Xu and his colleagues developed a fabrication method that produces individual palladium (Pd) catalytic nanoclusters inside porous organic cages [Yang et al., Nature Catalysis (2018), doi: https://doi.org/10.1038/s41929-018- 0030-8].
By creating catalytically active Pd nanoclusters inside organic molecular cages, aggregation and other problems are avoided, while making the entire surface available for catalytic reactions. The open channels of the porous organic cages make access easy for reactants.
“Not only do the Pd nanoclusters retain extremely high catalytic activity, they also show excellent solubility, dispersibility and stability,” says Xu.
The process used is a reverse double-solvents approach (RDSA), whereby a metal precursor in a water solution is combined with a hydrophobic organic liquid. The addition of a small amount of a hydrophobic solvent, containing the metal precursor, to a large amount of hydrophilic solvent drives the metal precursor into the hydrophobic cavities of, in this case, the reduced amine cage RCC3. The addition of NaBH4 reduces the metal precursor rapidly to create tiny Pd nanoclusters inside the cages (Fig. 1). The organic molecular cage is stable in air, water, and certain solvents, very flexible, and easy to fabricate.
“In particular, desolvated RCC3 are very ‘thirsty’ when it comes to hydrophobic molecules, which could benefit the diffusion of hydrophobic molecules into the cage cavities,” points out Xu.
The researchers believe that the majority of the Pd nanoclusters produced during the process (70%) are encapsulated inside the cages. They tested their caged catalysts with some classic reactions: hydrogen generation from ammonia borane, hydrogenation of nitroarenes, and the reduction of organic dyes, which is particularly important in environmental terms as organic dyes can be highly toxic pollutants. In each case, the caged Pd catalysts show very promising or significantly improved catalytic activity.
“Compared with traditional heterogeneous catalysts, our ultrafine Pd nanoclusters have excellent stability owing to the unique confinement of porous organic cages, while the open skeletons of the cage shells provide excellent accessibility to the Pd cores for reactants,” explains Xu.
The researchers believe that encapsulating metal nanoclusters within soluble, porous organic cages is a promising strategy for the development of advanced catalysts.
“This is exciting work,” comments Andy Cooper, professor of chemistry and director of the Materials Innovation Factory at the University of Liverpool in the UK. “The materials have high catalytic activities and clusters seem stable over several reaction cycles.”
However, he adds the caveat that it will be interesting to confirm where the Pd actually resides, whether it is indeed inside the cages, which could mean that the material is more like a Pd organic complex, or elsewhere.
Suresh Kalidindi of Poornaprajna Institute of Scientific Research in India agrees that confirmation is needed that the nanoclusters are residing within the cages.
“The role played by the organic cage is limited to restricting the size of Pd nanoparticles to <1 nm, but one clear novelty is the stabilization of sub-nanometer Pd nanoparticles, which is always a challenge in this size regime,” he points out. He also cautions that scaling up the approach looks difficult at this stage, which could limit its practical use.
This article was first published in Nano Today 20 (2018) 1-6.