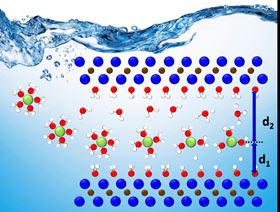
 Charged ions, shown in green, move into ultra-thin layers of MXene, shown as blue and brown dots, but are difficult to locate. The novel holistic approach to tracking the ions yielded knowledge that should be useful for developing improved energy-storage devices. Image: Nina Balke/ORNL, US Dept of Energy.
Charged ions, shown in green, move into ultra-thin layers of MXene, shown as blue and brown dots, but are difficult to locate. The novel holistic approach to tracking the ions yielded knowledge that should be useful for developing improved energy-storage devices. Image: Nina Balke/ORNL, US Dept of Energy.A team led by researchers at the US Department of Energy's Oak Ridge National Laboratory (ORNL) has developed a novel, integrated approach for tracking energy-transporting ions within an ultra-thin material. By unlocking the energy-storage potential of the material, this approach could help produce faster-charging, longer-lasting devices. The team reports its findings in a paper in Energy & Environmental Science.
Scientists have for a decade studied the energy-storage possibilities of an emerging class of two-dimensional (2D) materials – those constructed in layers that are only a few atoms thick – known as MXenes, pronounced 'max-eens'.
By integrating theoretical data from computational modeling of experimental data, the ORNL-led team found they could pinpoint the potential locations of a variety of charged ions in titanium carbide, the most studied MXene phase. Through this holistic approach, they were able to track and analyze the ions' motion and behavior from the single-atom to the device scale.
"By comparing all the methods we employed, we were able to form links between theory and different types of materials characterization, ranging from very simple to very complex over a wide range of length and time scales," said ORNL's Nina Balke, a co-author of the paper.
"We pulled all those links together to understand how ion storage works in layered MXene electrodes," she added. The study's results allowed the team to predict the material's capacitance, or its ability to store energy. "And, in the end, after much discussion, we were able to unify all these techniques into one cohesive picture, which was really cool."
Layered materials can enhance energy storage and power delivery because the gaps between the layers allow charged particles, or ions, to move freely and quickly. However, ions can be difficult to detect and characterize, especially in a confined environment with multiple processes at play. A better understanding of these processes could advance the energy storage potential of lithium-ion batteries and supercapacitors.
The team focused on the development of supercapacitors – devices that charge quickly for short-term, high-power energy needs. In contrast, lithium-ion batteries have a higher energy capacity and can provide electrical power for longer, but their rates of discharge – and therefore power levels – are lower.
MXenes have the potential to act as a bridge between supercapacitors and lithium-ion batteries, Balke said, producing fast-charging devices with greater, more efficient energy-storage capacity. This would benefit a range of applications, from electronics to electric vehicle batteries.
Using computational modeling, the team simulated the conditions of five different charged ions within MXene layers confined in an aqueous solution, or 'water shell'. This theoretical model is simple, but when combined with experimental data it created a baseline that provided evidence of where the ions within the MXene layers went and how they behaved in a complex environment.
"One surprising outcome was we could see, within the simulation limits, different behavior for the different ions," said ORNL theorist and co-author Paul Kent.
The team hopes their integrated approach can guide scientists toward future MXene studies. "What we developed is a joint model. If we have a little bit of data from an experiment using a certain MXene, and if we knew the capacitance for one ion, we can predict it for the other ones, which is something that we weren't able to do before," Kent said.
"Eventually, we'll be able to trace those behaviors to more real-world, observable changes in the material's properties," he added.
This story is adapted from material from ORNL, with editorial changes made by Materials Today. The views expressed in this article do not necessarily represent those of Elsevier. Link to original source.