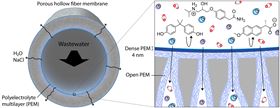
 Schematic of the new 'chimera' membrane.
Schematic of the new 'chimera' membrane.A new type of membrane that combines the best aspects of existing approaches could filter micropollutants like pharmaceuticals, pesticides, and plasticizers from drinking or waste water that other membranes cannot [Brinke et al., Applied Materials Today (2019), https://doi.org/10.1016/j.apmt.2019.100471].
“There is growing awareness and concern about the presence of organic micropollutants in our surface water, which are very small organic molecules stemming from medicines, pesticides, recreational drugs, and have the potential to cause long-term harm to humans and the environment,” explains Wiebe M. de Vos, who led the research at the University of Twente in the Netherlands.
Most organic micropollutants end up in our wastewater because current treatment plants are not designed or equipped for their removal. Some are potentially harmful and others, such as hormones and recreational drugs like cocaine, are of concern to the environment and fauna. Existing membranes struggle to remove this type of pollutant efficiently and economically, and typically produce highly saline waste streams that are difficult to treat.
“We have prepared a completely new type of membrane that allows water to be cleaned in a very efficient way,” says de Vos. “Our membrane is able to remove >98% of pesticides, hormones, remains of medicines, and leftovers of recreational drugs from the water… [with] removal efficiency higher than for commercial membranes.”
The new membrane consists of multiple very thin layers of polyelectrolytes (poly(acrylic acid), poly(allylamine hydrochloride) and poly(styrene sulfonate)) with different densities built up using a completely water-based coating procedure.
“We first adsorb a negatively charged polymer on a positive membrane, leading to a negatively charged layer,” explains de Vos. “On top of that we adsorb a positive polymer, then a negative one, then a positive one and so on… building up layer-by-layer.”
The asymmetric membrane includes a thicker but less dense polyelectrolyte layer, which prevents the formation of defects, and a very dense thin layer just a fraction of the thickness of conventional membranes (4 nm compared with 100 nm) that confers high selectivity.
“Our selective layer is 25 times thinner [than commercial membrane layers]! To our knowledge, [this is] the membrane with the thinnest top layer,” says de Vos. “At the same time, our approach is fully scalable and can easily be applied on large scales.”
While the membrane shows very high retention of micropollutants, it allows salt ions and water molecules to pass through more readily than commonly used reverse osmosis membranes. This avoids the creation of a highly saline waste stream, a problem that afflicts currently used membranes. Moreover, if all the salt is removed from water, some has to be added back in before it can be used for drinking or agriculture.
The team will now pilot the new membranes as post-treatment in a municipal wastewater treatment center to remove drugs and hormones before water enters the environment.