Cutaneous innervation is a fundamental element to skin physiopathology – it controls neurogenic inflammation, inflammatory responses originating in the afferent neurons, and it enhances wound healing. The generation of fully human innervated skin models is highly desirable, not only as an alternative to animal cells for cosmetic research and development purposes, but also to model human skin diseases in vitro. Due to the restricted access to human sensory neurons, up to date, innervated tissue-engineered skin models rely on the use of rodent sensory neurons, disregarding the possible existence of interspecies differences.
Muller and colleagues at Laval University (Université Laval) in Quebec, Canada, use human skin fibroblasts to produce induced pluripotent stem cells (iPSC) as the basis to generate different neuronal cell subtypes, sensory neurons and Schwann cells, and establish a 3D nerve network in vitro[Muller et al., Acta Biomaterialia(2018) doi: 10.1016/j.actbio.2018.10.011].
 A schematic of neuronal cell differentiation from induced pluripotent stem cells and the 3D human innervated tissue-engineered skin model
A schematic of neuronal cell differentiation from induced pluripotent stem cells and the 3D human innervated tissue-engineered skin modelThe researchers explain that efficient differentiation of sensory neurons in 3D is achieved by seeding of iPSC-derived neurons on a 3D collagen sponge at day 11 of differentiation, i.e., prior to neurite outgrowth, before the optimal maturation time observed in 2D (19 days). The proposed protocol overcomes limitations of cell purity and cell yields inherent to currently applied protocols and enables the establishment of a more physiologically-relevant 3D network, rather than a 2D monolayer.
Critical to neuroinflammation studies, the functional role of sensory neurons is demonstrated through the release of neuropeptides. Upon stimulation of specific receptors key to sensory physiology, the transient receptor potential (TRP) superfamily of cation channels, iPSC-derived sensory neurons exhibit the capacity to release neuropeptides Substance P and Calcitonin gene-related peptide.
Further innovative features of the system include prolonged neuronal culture (49 days in 3D versus 21 days in 2D) allowing neurons to reach a higher level of maturity.
The simultaneous culture of iPSC-derived neurons with iPSC-derived Schwann cells, which support neurons and nerve fiber survival, is necessary to induce neurite outgrowth in 3D. Co-culture of both cell subtypes allow the extension of neurites from neurons at the bottom of the tissue construct through the dermal compartment and formation of a nerve network that reaches the epidermis within the skin model.
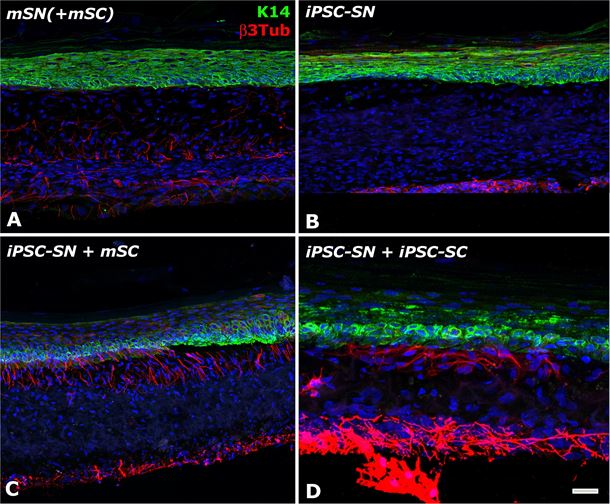 Immunofluorescence analysis of the capacity of the iPSC-derived neurons to form a 3D nerve network in the tissue-engineered skin. The construct made of keratinocytes (stained in green with Keratin 14), fibroblasts and mouse (A) or iPSC-derived (B-D) neurons (stained in red with beta 3 Tubulin) is co-cultured or not (B) with mouse (C) or iPSC-derived Schwann cells (D).
Immunofluorescence analysis of the capacity of the iPSC-derived neurons to form a 3D nerve network in the tissue-engineered skin. The construct made of keratinocytes (stained in green with Keratin 14), fibroblasts and mouse (A) or iPSC-derived (B-D) neurons (stained in red with beta 3 Tubulin) is co-cultured or not (B) with mouse (C) or iPSC-derived Schwann cells (D).The generation of such fully human innervated tissue-engineered skin can be achieved using patient-derived cells, with prospective applications in skin disease models for personalized medicine approaches. The researchers are now exploring different possibilities to create disease-specific skin models to study the regulation of neuroinflammation, including in psoriasis.