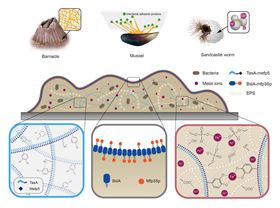
 (a) Illustration of natural marine adhesive. Left, barnacles using cement proteins containing amyloid-like structures. Middle, mussels using adhesive foot proteins that contribute to interfacial adhesion, ion-mediated self-coacervation and cohesion. Right, sandcastle worms using oppositely charged polyelectrolyte-induced complex coacervates. (b) Schematic of functional cellular glues based on engineered B. subtilis biofilms containing adhesive components inspired by the three natural marine systems. Top, illustration of an integrated biofilm-based functional cellular glue on a substrate, showing bacterial cells embedded inside a functionalized extracellular matrix rich in engineered amyloid structural proteins fused with a mussel foot protein, engineered biofilm surface proteins fused with an engineered mussel-derived peptide, exopolysaccaharides, and metal ions.
(a) Illustration of natural marine adhesive. Left, barnacles using cement proteins containing amyloid-like structures. Middle, mussels using adhesive foot proteins that contribute to interfacial adhesion, ion-mediated self-coacervation and cohesion. Right, sandcastle worms using oppositely charged polyelectrolyte-induced complex coacervates. (b) Schematic of functional cellular glues based on engineered B. subtilis biofilms containing adhesive components inspired by the three natural marine systems. Top, illustration of an integrated biofilm-based functional cellular glue on a substrate, showing bacterial cells embedded inside a functionalized extracellular matrix rich in engineered amyloid structural proteins fused with a mussel foot protein, engineered biofilm surface proteins fused with an engineered mussel-derived peptide, exopolysaccaharides, and metal ions.Researchers have developed a ‘living glue’ inspired by the ability of marine organisms like barnacles and mussels to stick tenaciously to rocks [Zhang et al., Materials Today (2019), https://doi.org/10.1016/j.mattod.2018.12.039]. A flexible natural adhesive, which could work underwater or in highly damp or humid conditions, could bond tissue or wounds back together or find use in electronic skin or ion-exchange battery separators.
But although artificial adhesives that mimic the adherent properties of marine organisms have been reported, they lack the self-regeneration and environmental responsiveness of living systems. So Chao Zhong of ShanghaiTech University and his colleagues from the University of Chinese Academy of Sciences in Beijing, Shanghai Institute of Organic Chemistry, Shanghai Institute of Applied Physics, Shanghai Jiao Tong University, and Massachusetts Institute of Technology went back to nature.
“By leveraging tools from genetic engineering and materials science, we can turn bacterial biofilms into sticky glues by tuning and modifying their matrix components,” explains Zhong, who led the research.
A biofilm is a colony of bacteria and other microorganisms forming a slimy, sticky mass on surfaces that can often, in the form of dental plaques or on medical equipment, be harmful. But Zhong and his team decided to turn the adherent nature of biofilms to their advantage.
“We developed a living cellular glue based on engineered Bacillus subtilis biofilms that recapitulates and integrates the adhesive characteristics of three different biological adhesion systems: barnacle cements, mussel foot proteins, and sandcastle worm coacervates,” he told Materials Today.
The researchers based their living glue on biofilms of the rod-shaped bacteria B. subtilis because it only has one outer membrane. The extracellular matrix of B. subtilis contains TasA amyloid fibers, which readily attach to other peptides or proteins, hydrophobic surface layer proteins BslA, and exopolysaccharides (EPS). To start with, the researchers functionalized the amyloid proteins with a mussel foot protein that increases adhesion and then added a peptide to the surface layer protein that boosts the tendency of liquid phases to form polymeric droplets (a process known as coacervation), which helps prevent water penetration. Finally, the team shows that curing the engineered biofilms with different metal ions such as Ca2+, Mg2+, and Fe3+ improves adhesion even further.
“Our glue is the first demonstration of an engineered ‘living’ glue with strong underwater adhesion performance, environmental tolerance, and regenerative capacity,” says Zhong.
Moreover, because the glue is alive, it can be directed to evolve to improve its performance still further or in response to particular environmental conditions. This strategy could improve the adhesion strength of the glue, which the researchers admit is lower than some highly engineered artificial adhesives. The researchers now plan to introduce new genetically encoded adhesive components into the system.