As hard as diamond and as flexible as plastic, highly sought-after diamond nanothreads would be poised to revolutionize our world – if they weren’t so difficult to make.
Now, a team of scientists led by Samuel Dunning and Timothy Strobel at the Carnegie Institution for Science has developed an original technique that predicts and guides the ordered creation of strong, yet flexible, diamond nanothreads, surmounting several existing challenges. This innovation will make it easier for scientists to synthesize these nanothreads – an important step toward applying the material to practical problems in the future. The scientists report their work in a paper in the Journal of the American Chemical Society.
Diamond nanothreads are ultra-thin, one-dimensional carbon chains, tens of thousands of times thinner than a human hair. They are often created by compressing smaller carbon-based rings together to form the same type of bond that makes diamonds the hardest mineral on our planet.
However, instead of the 3D carbon lattice found in normal diamond, the edges of these threads are ‘capped’ with carbon-hydrogen bonds, which make the whole structure flexible. “Because the nanothreads only have these bonds in one direction, they can bend and flex in ways that normal diamonds can’t,” explains Dunning.
Scientists predict that the unique properties of carbon nanothreads will lead to a range of useful applications, from providing sci-fi-like scaffolding for space elevators to creating ultra-strong fabrics. However, scientists have had a hard time creating enough nanothread material to actually test their proposed superpowers.
“If we want to design materials for specific applications,” says Dunning, “it's essential for us to precisely understand the structure and bonding of the nanothreads we're making. This thread directing method really allows us to do that!”
One of the biggest challenges in producing nanothreads is getting the carbon atoms to react in a predictable way. In nanothreads made from benzene and other six-atom rings, each carbon atom can undergo chemical reactions with different neighbors. This leads to many possible reactions competing with one another and many different nanothread configurations. This uncertainty is one of the biggest hurdles scientists face in synthesizing nanothreads with a precise chemical structure.
Dunning’s team determined that adding nitrogen to the ring in place of carbon might help guide the reaction down a predictable pathway. To test this idea, they stated with pyridazine – a six-atom ring made up of four carbon atoms and two nitrogen atoms – and began developing a computer model. Working with Bo Chen at the Donostia International Physics Center in Spain and Li Zhu, an assistant professor at Rutgers University, Dunning simulated how pyridazine molecules behave at high pressure.
“In our system, we use two nitrogen atoms to remove two possible reaction sites from the ring system,” says Dunning. “This dramatically reduces the number of possible reactions.”
After running several computer simulations showing successful nanothread formation at high pressure, the scientists were ready to take the experiment into the lab. They loaded a drop of pyridazine into a diamond anvil cell – a device that allows scientists to produce extreme pressures by compressing samples between the tiny tips of more traditional diamonds. Using infrared spectroscopy and X-ray diffraction, they monitored changes in the pyridazine’s chemical structure up to about 300,000 times normal atmospheric pressure, looking for the creation of new bonds.
When they saw the bonds forming, they realized they had successfully predicted and created the first pyridazine diamond nanothread in the lab.
“Our reaction pathway produces an incredibly orderly nanothread,” says Dunning. “The ability to incorporate other atoms into the nanothread backbone, guide the reaction and understand the nanothread’s chemical environment will save researchers invaluable time in developing nanothread technology.”
The process of using these non-carbon atoms to guide the formation of nanothreads, which Dunning calls ‘thread directing’, represents a significant step towards a future where scientists can predictably create these materials and use them for advanced applications. Now that this synthetic strategy has been discovered, Dunning plans to identify and test the many possible nanothread precursors.
He also can’t wait to start putting the pyridazine nanothreads through their paces. “Now that we know we can make this material, we need to start making enough to learn enough to determine mechanical, optical and electronic properties.”
This story is adapted from material from the Carnegie Institution for Science, with editorial changes made by Materials Today. The views expressed in this article do not necessarily represent those of Elsevier. Link to original source.
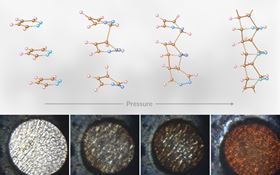 The starting sample of pyridazine changes under pressure as diamond nanothread formation progresses. The first and last images on the bottom row show that there is a permanent color change after thread formation. The images don’t show individual threads but ‘bulk’ samples of pyridazine during compression, each around 40µm thick with a diameter of 180µm. Image: Samuel Dunning.
The starting sample of pyridazine changes under pressure as diamond nanothread formation progresses. The first and last images on the bottom row show that there is a permanent color change after thread formation. The images don’t show individual threads but ‘bulk’ samples of pyridazine during compression, each around 40µm thick with a diameter of 180µm. Image: Samuel Dunning.