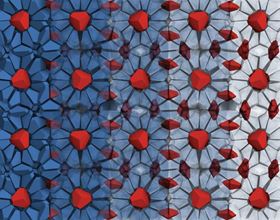
 The cages of the host network of bipyramid particles are shown in blue on the left side, becoming increasingly transparent toward the right. The red bipyramid particles are guest particles, trapped in the cages of the clathrate structure. Image: Sangmin Lee, Glotzer Group.
The cages of the host network of bipyramid particles are shown in blue on the left side, becoming increasingly transparent toward the right. The red bipyramid particles are guest particles, trapped in the cages of the clathrate structure. Image: Sangmin Lee, Glotzer Group.Cage structures made with nanoparticles could offer a route toward fabricating organized nanostructures with mixed materials, and researchers at the University of Michigan have now used computer simulations to show how this can be done.
Their work, reported in a paper in Nature Chemistry, could open new avenues for photonic materials that manipulate light in ways that natural crystals can't. It also showcased an unusual effect that the team is calling entropy compartmentalization.
"We are developing new ways to structure matter across scales, discovering the possibilities and what forces we can use," said Sharon Glotzer, chair of chemical engineering at the University of Michigan, who led the study. "Entropic forces can stabilize even more complex crystals than we thought."
While entropy is often explained as disorder in a system, it more accurately reflects the system's tendency to maximize its possible states. Often, this ends up as disorder in the colloquial sense. Oxygen molecules don't huddle together in a corner – they spread out to fill a room. But if you put them in the right-size box, they will naturally order themselves into a recognizable structure.
Nanoparticles do the same thing. Previously, Glotzer's team had shown that bipyramid nanoparticles – like two short, three-sided pyramids stuck together at their bases – will form structures resembling fire ice if you put them into a sufficiently small box.
Fire ice is made up of cages of water molecules that form around methane molecules, allowing it to burn and melt at the same time. This substance is found in abundance under the ocean floor and is an example of a clathrate. Clathrate structures are under investigation for a range of applications, such as trapping and removing carbon dioxide from the atmosphere.
Unlike water clathrates, previous nanoparticle clathrate structures had no gaps for filling with other materials that might provide new and interesting possibilities for altering the structures’ properties. The team wanted to change that.
"This time, we investigated what happens if we change the shape of the particle," said Sangmin Lee, a recent doctoral graduate in chemical engineering and first author of the paper. “We reasoned that if we truncate the particle a little, it would create space in the cage made by the bipyramid particles.”
Using computer simulations, Lee experimented with taking the three central corners off each bipyramid. This allowed him to discover the sweet spot where spaces began appearing in the structure but the sides of the pyramids were still intact enough that they didn't start organizing in a different way. The spaces filled in with more truncated bipyramids when they were the only particle in the system. When a second shape was added, however, it became the trapped guest particle.
Glotzer has ideas for how to create selectively sticky sides that would allow different materials to act as cage and guest particles. In this case, however, there was no glue holding the bipyramids together. Instead, the structure was completely stabilized by entropy.
"What's really fascinating, looking at the simulations, is that the host network is almost frozen,” she said. “The host particles move, but they all move together like a single, rigid object, which is exactly what happens with water clathrates. But the guest particles are spinning around like crazy – like the system dumped all the entropy into the guest particles."
This was the system with the most degrees of freedom that the truncated bipyramids could construct in a limited space, but nearly all the freedom belonged to the guest particles. The methane in the water clathrates rotates too, the researchers say. What's more, when they removed the guest particles, bipyramids that had been part of the networked cage structure were thrown into the cage interiors – it was more important to have spinning particles available to maximize the entropy than to have complete cages.
"Entropy compartmentalization. Isn't that cool? I bet that happens in other systems too—not just clathrates," Glotzer said.
This story is adapted from material from the University of Michigan, with editorial changes made by Materials Today. The views expressed in this article do not necessarily represent those of Elsevier. Link to original source.