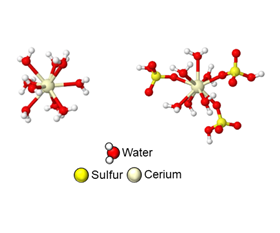
 When the cerium atom is short three electrons, it is surrounded by water molecules. But when it gives up a fourth electron, some water molecules shift out of the way to let in sulfates. This dance costs energy, but understanding that energy loss paves the way for more efficient cerium batteries. Image: Dylan Herrera, Goldsmith Lab.
When the cerium atom is short three electrons, it is surrounded by water molecules. But when it gives up a fourth electron, some water molecules shift out of the way to let in sulfates. This dance costs energy, but understanding that energy loss paves the way for more efficient cerium batteries. Image: Dylan Herrera, Goldsmith Lab.A team led by researchers at the University of Michigan (U-M) has discovered an explanation for why flow batteries that use the metal cerium in a sulfuric acid electrolyte fall short on voltage. This finding, reported in a paper in JACS Au, could pave the way for better battery chemistry.
Flow batteries are one of the technologies under consideration for storing intermittent sources of renewable electricity, such as solar and wind power. They can bank large quantities of energy by keeping the chemical potential in liquid form, with two cerium-containing electrolytes that flow through porous electrodes for charging and discharging. Cerium helps the flow battery to store energy at a relatively high voltage, meaning more energy per metal ion, and at low cost.
One of the challenges with cerium is figuring out how to make electric charges transfer to and from the electrode efficiently. On its way through the positive electrode, cerium either picks up or drops off an electron, depending on whether the battery is charging or discharging. But in a sulfuric acid electrolyte, the cerium doesn't pick up and drop off the electron as quickly as expected, meaning energy is wasted. The team has now discovered this is due to a complicated dance that the water molecules and sulfate molecules do around the cerium.
"Through this study, we have a better understanding of how cerium ions behave in acidic electrolytes during charge transfer," said Cailin Buchanan, a recent U-M doctoral graduate in chemical engineering and first author of the paper. "This understanding will help us and future researchers to design more efficient cerium-based batteries that have less voltage loss during charging and discharging."
The team took a close look at what was happening when cerium picked up and dropped off electrons, using X-ray absorption to keep an eye on the bonds and associations between the cerium, sulfates and water. These experiments were done at Argonne National Laboratory. They followed those measurements with computer simulations, led by Bryan Goldsmith, an assistant professor of chemical engineering at U-M.
"We find that when cerium is short three electrons, it is only surrounded by water molecules, while when it gives up that fourth electron, sulfate or bisulfate ions are hanging off the cerium ion," said Nirala Singh, U-M assistant professor of chemical engineering and corresponding author of the paper, who led the experiment.
"As a result of this, when we oxidize cerium by taking away that electron, or reduce it by giving the electron back, both an electron transfer has to occur and the molecules around it have to rearrange."
By understanding the energy associated with this structural rearrangement, the researchers were able to explain why the reaction is asymmetrical, with the oxidation and reduction reactions behaving differently. Because of this, the go-to theory for predicting the rates of electron transfer, known as the Marcus theory, isn't enough. Instead, the team found that it's possible to use the Marcus theory to figure out the electron transfer and then add in the effects of the rearrangement in a two-step process.
"The uneven complexation between the oxidized and reduced forms of cerium causes reaction rates to slow down, and this knowledge will inform electrolyte design strategies for cerium or other similar flow batteries," Singh said.
Using the team's two-step method, researchers will now be able to identify electrolytes that have fast reaction rates, yielding high efficiencies. Ultimately, the goal is to use electrolytes that don't store different amounts of energy in the complexes that form around the oxidized or reduced cerium ion.
In addition to opening new avenues in grid-scale energy storage, this discovery could improve other chemical processes that rely on cerium, such as manufacturing carbon-based products and treating wastewater.
This story is adapted from material from the University of Michigan, with editorial changes made by Materials Today. The views expressed in this article do not necessarily represent those of Elsevier. Link to original source.