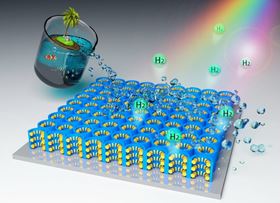
Researchers at the University of central Florida, USA, have shown that hydrogen gas can be efficiently released from seawater using a hybrid nanomaterials, a photocatalyst, comprising an ultrathin film of titanium dioxide with nanocavity indentations coated with molybdenum disulfide. The technology could reduce the energy cost of hydrolyzing water sufficiently to make the process viable for obtaining the gas for use in future fuel cells. The work is the culmination of a decade's worth of investigation by Yang Yang and colleagues and details are published this month. [Guo et al.,Energy Environ. Sci., (2017) DOI: 10.1039/C7EE02464A]
Seawater and sunny climes would seem a perfect combination for generating hydrogen sustainably as a feedstock for fuel cells. A photocatalyst would be driven by sunlight and seawater represents essentially an unlimited supply of hydrogen. Unfortunately, seawater is not only corrosive but carries with it biomass, neither properties are conducive to a long-lived and efficient catalytic system. Yang's new nanomaterial, however, is resistant to corrosion and also a lot more efficient at capturing the energy of solar photons across a broad band of wavelengths.
"We've opened a new window to splitting real water, not just purified water in a lab," Yang explains. "This really works well in seawater." The team etched tiny nanocavities into the surface of an ultrathin film of titanium dioxide, which is already a widely used photocatalyst. To endow their material with the ability to process seawater, the team applied nanoflakes of molybdenum disulfide, one of a growing number of two-dimensional materials just a single atom thick, to the nanocavities.
By adjusting the density of sulphur vacancies in the nanoflakes within the cavities, the material can convert energy from the ultraviolet-visible (UV-vis) to near-infrared (NIR) wavelengths. This makes the photocatalyst at least twice as efficient as current materials. In many situations, producing a chemical fuel from solar energy is more useful than simply generating electricity with solar panels. The team hopes to eventually commercialize their photocatalyst and is currently optimizing fabrication with a view to scaling up the process for industrial application.
"As an innovative exploration, our study demonstrates that the photocatalytic activities of non-metal, earth-abundant materials can be enhanced with plasmonic effects, which may serve as an excellent catalytic agent for solar energy conversion to chemical fuels," the team reports.
David Bradley blogs at Sciencebase Science Blog and tweets @sciencebase, he is author of the popular science book "Deceived Wisdom".